Tau pre-formed fibrils (PFFs) are commonly employed to simulate the prion-like spread of tau pathology in Alzheimer's disease (AD) and other tauopathies.
Human induced pluripotent stem cell (iPSC)-derived cerebral organoids offer a physiologically realistic three-dimensional (3D) platform that replicates important elements of human cortex development and organization.
This study established an in vitro model by combining tau PFFs and brain organoids to examine tau pathogenesis and potential therapeutic approaches.
Introduction
Alzheimer's disease is a progressive neurodegenerative ailment marked by amyloid plaques and neurofibrillary tangles, which cause synapse loss and cognitive deterioration. Pathological tau, particularly in its fibrillar form, travels through the brain in a prion-like manner, spawning aggregation of endogenous tau in recipient neurons.
This propagation is strongly associated with disease progression in AD and other tauopathies, and it is typically modeled using recombinant Tau PFFs.
Cerebral organoids generated from human iPSCs mimic key aspects of early human brain development, such as cortical layer architecture, various neuronal cell types, and synaptic function.
Their 3D structure and human origin make them an excellent platform for simulating complex neurodegenerative processes. This study created a co-culture paradigm by incorporating tau PFFs into iPSC-derived cerebral organoids to mimic human tau disease in vitro.
This model induces typical clinical traits such as tau hyperphosphorylation, aggregation, and glial activation, and it serves as a human-relevant platform for mechanistic research and therapeutic screening.
Results
- Cerebral organoids contain abundant mature neurons and glial cells
To create a physiologically mature organoid model, human iPSC-derived cerebral organoids (Cat. No. CIPO-BWL002K) were created with a commercial differentiation kit (Cat. No. RIPO-BWM001K) and cultured for over 100 days.
These organoids had well-organized laminar structures similar to cortical layers II-VI and expressed key neuronal and glial markers, such as glutamatergic, GABAergic, and dopaminergic neurons, as well as astrocytes and oligodendrocytes (Figure 1), indicating advanced maturation and cellular heterogeneity.
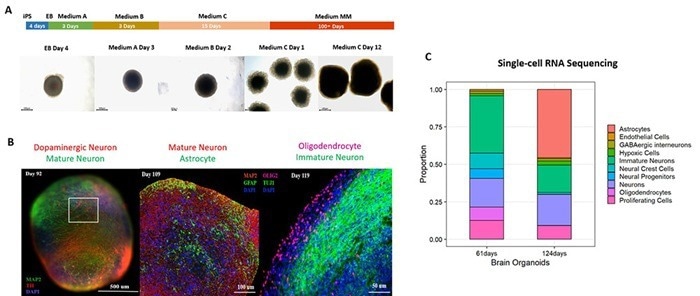
Figure 1. (A) Schematic of the cerebral organoid differentiation process. (B) Immunostaining and (C) single-cell RNA sequencing confirm robust expression of mature neuronal and glial markers in 100+ day cerebral organoids. Image Credit: ACROBiosystems
- Tau PFF treatment induces cell apoptosis in cerebral organoids
To evaluate the effect of exogenous Tau seeding, day 100+ organoids were treated with recombinant Tau PFFs (Cat. No. TAU-H5113 / TAU-H5116) at 10 μg/mL or 100 μg/mL.
The cultures were kept for ten days, with medium changes every five days. After five days of treatment, apoptotic cells were seen with visible cellular debris surrounding the organoids at 100 μg/mL, showing a concentration-dependent cytotoxic impact of Tau PFFs (Figure 2).
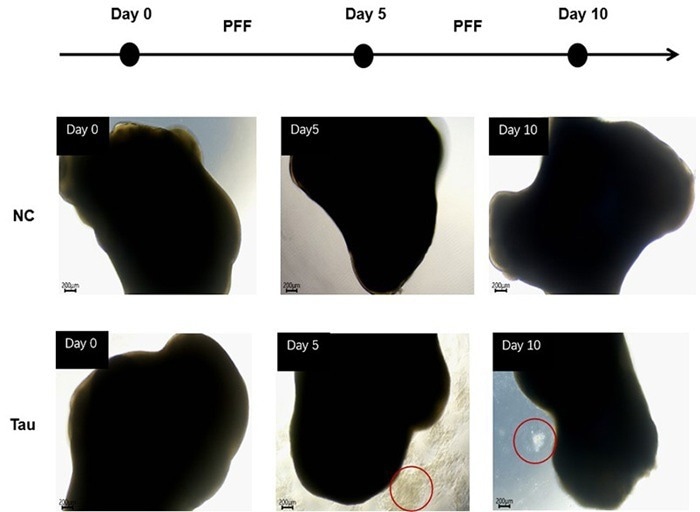
Figure 2. Cell apoptosis was observed after 5 days of Tau PFF treatment. Image Credit: ACROBiosystems
- Tau PFF treatment increases phosphorylated Tau levels in a dose-dependent manner
To examine the influence of Tau PFFs on tau pathology, cerebral organoids were co-cultured with recombinant Tau PFFs and measured phospho-Tau accumulation. Immunostaining demonstrated considerable increases in pTau181 (Figure 3) and AT8 (Figure 4) after treatment.
Western blot analysis corroborated the concentration-dependent increase in pTau217 levels (Figure 5). These findings suggest that Tau PFFs cause phosphorylation and seed-dependent proliferation of endogenous Tau, which replicates key clinical characteristics of AD1-5.
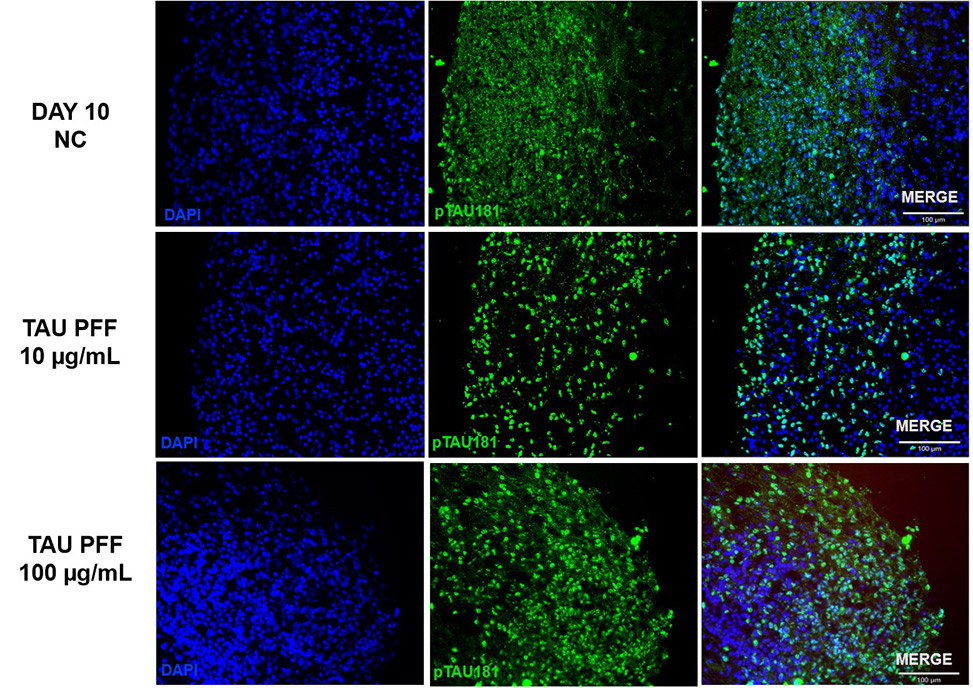
Figure 3. The expression of pTau181 increased following Tau PFF treatment. Image Credit: ACROBiosystems
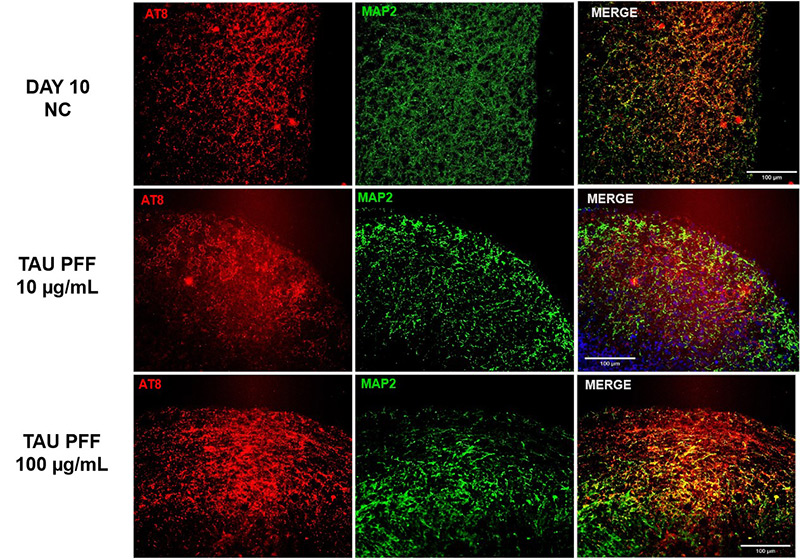
Figure 4. The expression of AT8 increased following Tau PFF treatment. Image Credit: ACROBiosystems
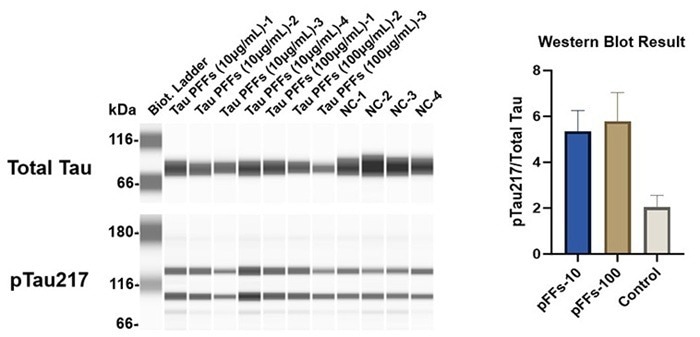
Figure 5. Tau PFFs increase Tau phosphorylation at Thr217 site. Image Credit: ACROBiosystems
- Tau PFF treatment induces astrocyte activation in cerebral organoids
The expression of GFAP and S100β in cerebral organoids was measured to quantify astrocyte activation after Tau PFF administration.
GFAP expression increased dramatically following treatment, especially at 100 μg/mL (Figure 6). Signals extended from the organoid surface to deeper layers, indicating reactive astrocytosis. S100β, a CNS-specific astrocyte marker, demonstrated higher expression and deeper tissue penetration at 100 μg/mL (Figure 7).
These results indicate that high-dose Tau PFFs may cause significant astrocyte activation and early neuroinflammatory reactions in cerebral organoids.5-6
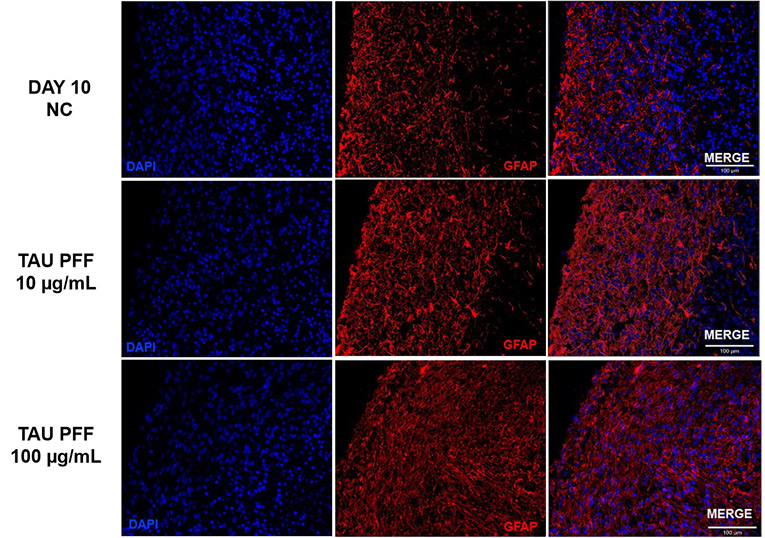
Figure 6. Tau PFF treatment increased GFAP expression, leading to wider distribution and deeper penetration into the brain organoid, indicating enhanced astrocyte activation. Image Credit: ACROBiosystems
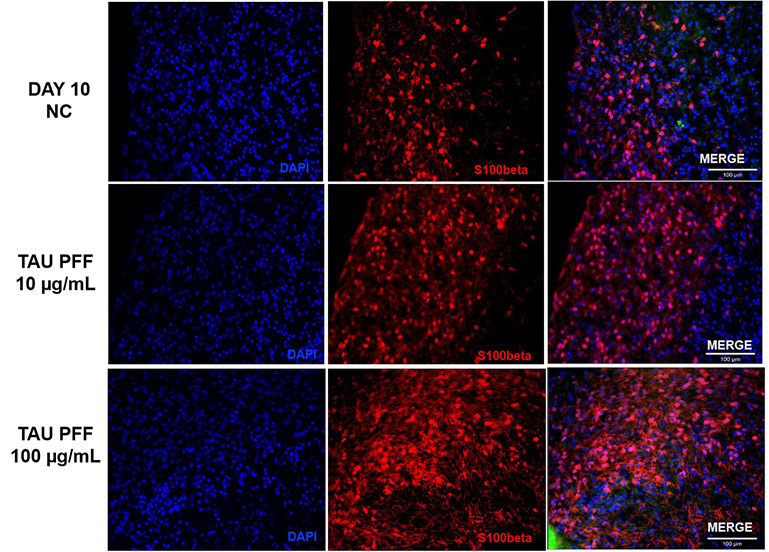
Figure 7. S100β expression also increased after Tau PFF treatment, likely due to astrocyte activation and potential involvement in neuroinflammatory responses. Image Credit: ACROBiosystems
- Tau PFF treatment induces neuroinflammation and impairs antioxidant defenses in cerebral organoids
To evaluate Tau PFFs' neuroinflammatory and oxidative effects, cytokine expression and antioxidant responses were measured in cerebral organoids. Tau PFF therapy increased pro-inflammatory cytokines IL-1β, IL-6, and IL-8, while decreasing SOD2, an important mitochondrial antioxidant enzyme.
Elevated IL-1β levels may trigger NF-κB signaling in glial cells, leading to increased release of IL-6 and IL-8.
This chronic inflammatory environment may cause neuronal damage and increased oxidative stress via glia-derived reactive oxygen species (ROS). Downregulation of SOD2 may exacerbate mitochondrial dysfunction by allowing superoxide radicals to accumulate.7-9
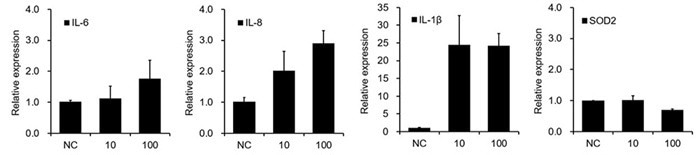
Figure 8. Tau PFF induces neuroinflammation and disrupts antioxidant balance. Image Credit: ACROBiosystems
Conclusion
This study develops a robust Tau PFF-cerebral organoid co-culture model that accurately replicates major clinical characteristics of Alzheimer's disease and similar tauopathies.
Exogenous Tau PFFs successfully initiate pathology inside cerebral organoids by causing dose-dependent hyperphosphorylation of Tau at disease-relevant sites such as pTau181, pTau217, and AT8.
Tau PFF exposure significantly activates astrocytes, as shown by increased expression of GFAP and S100β. The accompanying degenerative alterations progressed from the organoid surface to deeper cortical regions, matching the spatial pattern of astrocyte activation in different tissue layers.
Activated glia cells can cause neuroinflammation and susceptibility by upregulating pro-inflammatory cytokines such IL-1β, IL-6, and IL-8 through NF-κB-mediated pathways.
The downregulation of the mitochondrial antioxidant enzyme SOD2 leads to increased ROS buildup and mitochondrial malfunction, creating a vicious cycle of inflammation and oxidative stress that exacerbates neuronal damage and death.
Human-derived organoids fill the gap between typical 2D in vitro systems and animal models, offering a pathologically relevant platform for investigating Tau aggregation and propagation, neuroinflammation-oxidative stress connections, and glia-neuron crosstalk.
It also allows for therapeutic screening for drugs that target Tau seeding, inflammatory pathways (e.g., IL-1β/NF-κB), or mitochondrial redox imbalance (e.g., SOD2 modulators). This system provides a valuable resource for mechanistic research and translational studies in neurodegenerative diseases.
Methods and materials
- Samples and reagents
- PFFs preparation
Tau PFFs were created by incubating recombinant human Tau monomers at 37 °C with continual agitation for 7 days to promote fibrillation. The production of fibrils was confirmed by the Thioflavin T (ThT) fluorescence assay and transmission electron microscopy (TEM). The resultant PFFs were aliquoted and kept at -80 °C.
Prior to use, PFFs were quickly sonicated to produce shorter fragments suited for cellular absorption. Sonicated PFFs were diluted to final concentrations of 10 or 100 μg/mL in cerebral organoid culture media. The media were changed every five days, and the treatment was continued for ten days.
- Single-cell RNA sequencing
A third-party service performed single-cell RNA sequencing.
- Immunofluorescence staining and simple western analysis
Cerebral organoids were fixed in 4 % paraformaldehyde, cryoprotected in 30 % sucrose, and then sectioned with a cryostat. The sections were permeabilized, blocked, and treated with primary antibodies overnight at 4 °C.
After washing, fluorescent secondary antibodies were applied for two hours at room temperature. Nuclei were counterstained with DAPI, and slides were mounted using ProLong™ Gold Antifade Mountant. Imaging was done using a Zeiss Axio Observer Apotome microscope.
Organoids were lysed in RIPA buffer containing protease and phosphatase inhibitors to determine protein levels.
The Simple Western Jess method (ProteinSimple, Bio-Techne) was used to analyze the protein expression levels of total Tau and phosphorylated Tau (pTau217). Sample separation, immunodetection, and chemiluminescent signal capture were done in accordance with the manufacturer's instructions.
GAPDH was employed as the loading control. The signal intensities were evaluated and normalized using Bio-Techne's Compass for Simple Western software.
- Real-time quantitative polymerase chain reaction (RT-qPCR)
The FastPure Cell/Tissue TotalRNA Isolation Kit V2 was used to isolate total RNA, and the HiScript ll RT SuperMix for qPCR (+gDNA wiper) (Vazyme) was used to produce template complementary DNA by reverse transcription. qPCR was carried out with the Tag Pro Universal SYBRqPCR Master Mix (Vazyme).
Reference
- Rawat, P., et al. (2022). Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. International Journal of Molecular Sciences, 23(21), p.12841. https://doi.org/10.3390/ijms232112841.
- Kaufman, S.K., et al. (2016). Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron, (online) 92(4), pp.796–812. https://doi.org/10.1016/j.neuron.2016.09.055.
- Sanders, D.W., et al. (2014). Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron, (online) 82(6), pp.1271–88. https://doi.org/10.1016/j.neuron.2014.04.047.
- Liu, F., et al. (2019). Advances in Cerebral Organoid Systems and their Application in Disease Modeling. Neuroscience, (online) 399, pp.28–38. https://doi.org/10.1016/j.neuroscience.2018.12.013.
- Vogel, J.W., et al. (2020). Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nature Communications, (online) 11(1), p.2612. https://doi.org/10.1038/s41467-020-15701-2.
- Michetti, F., et al (2023). The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. International Journal of Molecular Sciences, (online) 24(11), pp.9605–9605. https://doi.org/10.3390/ijms24119605.
- Kummer, K.K., et al. (2021). Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine, 144, p.155582. https://doi.org/10.1016/j.cyto.2021.155582.
- Vilotić, A., et al. (2022). IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. International Journal of Molecular Sciences, (online) 23(23), p.14574. https://doi.org/10.3390/ijms232314574.
- Flynn, J.M. and Melov, S. (2013). SOD2 in Mitochondrial Dysfunction and Neurodegeneration. Free radical biology & medicine, (online) 62. https://doi.org/10.1016/j.freeradbiomed.2013.05.027.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.