Pfizer's integrin αvβ6-targeting Antibody-Drug Conjugate (ADC) candidate, Sigvotatug Vedotin (PF-08046047, also known as SGN-B6A), has progressed to Phase III clinical trials for PD-L1 high-expressing NSCLC.
With its promising anti-cancer activity, Sigvotatug Vedotin has emerged as a potential "First-in-Class" integrin β6 ADC therapy in 2025.
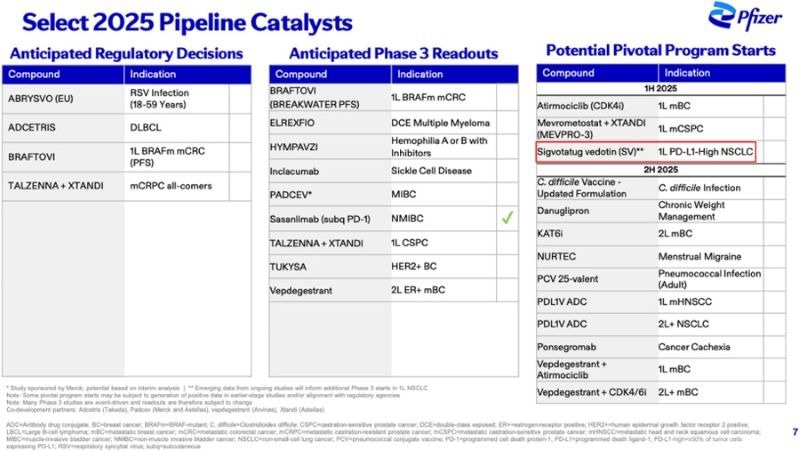
Pfizer top pipeline catalysts in 2025 include Sigvotatug Vedotin. Image Credit: ACROBiosystems
Sigvotatug Vedotin: Mechanism and clinical progress
Sigvotatug Vedotin is an ADC that targets integrin αvβ6-positive tumor cells. It has an engineered antibody that ensures high specificity, while a protease-cleavable linker delivers the microtubule inhibitor MMAE (monomethyl auristatin E).
When internalized, MMAE disrupts microtubules, inhibits cell division, and eventually leads to tumor cell death.
Pfizer presented Phase I clinical data of Sigvotatug Vedotin which showed a confirmed objective response rate (cORR) of 19.5 %, with a median duration of response (mDOR) of 8.4 months at ASCO in 2024.
In non-squamous, non-small cell lung cancer (NSCLC) patients who had not previously received taxane therapy, the drug showed strong anti-tumor activity with a cORR increased to 32.5 %, and an mDOR of 11.6 months.
As a result of these encouraging results, Sigvotatug was accelerated into Phase III trials, bypassing Phase II altogether.
Integrin: Key players in tumor development and metastasis
Integrins are critical in cancer progression, influencing proliferation, angiogenesis, invasion, metastasis, immune evasion, metabolic regulation, apoptosis resistance, and inflammatory responses.
By interacting with the extracellular matrix and signaling pathways, integrins promote tumor cell growth and neovascularization. In doing so, migratory capacity is enhanced for distant colonization.
Integrins also modulate the immune microenvironment and play a key role in drug resistance, making them significant in cancer research and targeted therapy development.
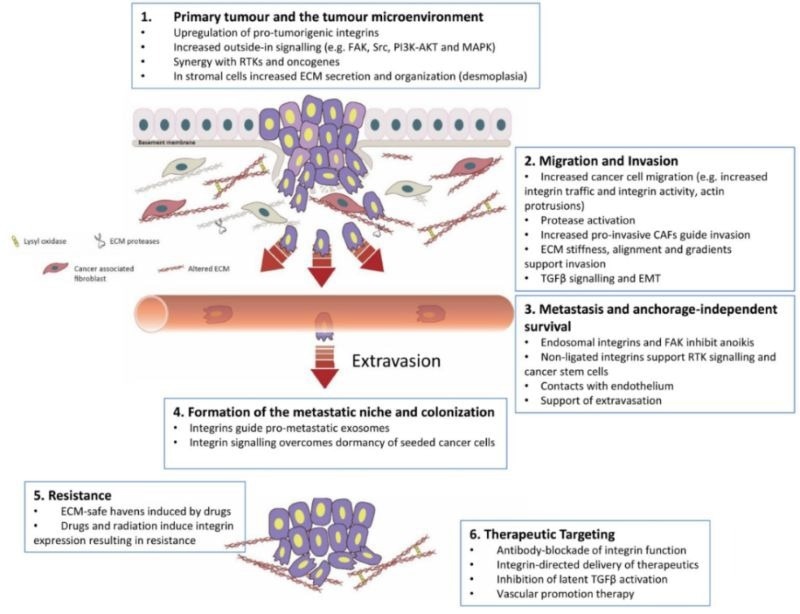
Integrin-mediated tumor cell migration process. Image Credit: ACROBiosystems
Early integrin-targeted therapeutics primarily focused on small-molecule inhibitors and blocking antibodies. However, these agents do not directly induce tumor cell death and had limited efficacy as standalone therapies.
Clinical data has shown that despite drugs such as Intetumumab, Abituzumab, and Cilengitide demonstrated favorable safety profiles, their therapeutic benefits remained modest.
Research has shifted towards enhancing tumor-killing activity as a result, leading to the development of these next-generation therapies targeting Integrin αvβ6 and Integrin αvβ8.
These include ADCC-enhanced antibodies, ADCs, and T-cell engagers (TCEs), all of which represent a substantial advancement in integrin-targeted cancer therapy.
Innovative integrin-targeted therapies in development
Madwell 2MW4991 is an ADCC-enhanced integrin αvβ8-specific blocking antibody with sub-nM affinity for αvβ8 and no binding to other integrins.
It blocks αvβ8-mediated TGF-β release and exhibits potent anti-tumor activity in tumor models like CT26 and EMT6. Madwell 2MW4991 promotes immune cell infiltration and enhances the sensitivity of immune-excluded tumors to PD-1 inhibitors.
Non-primate model studies of this drug show its favorable safety profile and metabolic stability.1
Pfizer SGN-B6A is an ADC developed by Seagen (now part of Pfizer), targeting integrin αvβ6.
SGN-B6A combines an αvβ6 antibody with MMAE and a mc-vc linker, featuring a drug-to-antibody ratio (DAR) of four. This drug binds specifically to integrin αvβ6 and demonstrates substantial internalization within four hours.
Integrin αvβ6 is highly expressed in various solid tumors, including NSCLC, head and neck cancer, and esophageal cancer, and is associated with poor prognosis.
Using a protease-cleavable linker to conjugate the αvβ6 antibody with MMAE, SGN-B6A shows substantial anti-tumor activity. Preclinical studies have shown efficacy across multiple tumor models.2
Harpoon Therapeutics CD3 x ITGB6 TCE is a bispecific TCE being developed by Harpoon Therapeutics based on the ProTriTAC™ platform, which targets integrin αvβ6 and CD3.
This TCE aims to selectively kill αvβ6-expressing tumor cells by recruiting and activating T cells, while optimizing its half-life to enhance efficacy and reduce toxicity. Preclinical studies have demonstrated significant anti-tumor activity in various high αvβ6-expressing tumor models.3
Comprehensive integrin-targeted drug development solutions
To address the development needs for integrin-targeted therapies in oncology and non-oncology indications, ACROBiosystems has launched a comprehensive range of high-quality biopharmaceutical development tools.
These tools support drug development applications from the molecular to the cellular level, ensuring precision and efficacy throughout the process.
- 24 integrin heterodimer subtype proteins: These are high-quality recombinant proteins, including heterodimers and monomers, exhibiting a high purity and activity for immune assays, antibody screening, and functional validation of candidate drugs.
- Integrin α4β7: MAdCAM-1 [Biotinylated] inhibitor screening ELISA kit: This kit detects inhibitors of ITG α4β7-MAdCAM-1 binding, effectively quantifying the inhibitory effects of candidate molecules on the binding between ITG α4β7 and its receptor MAdCAM-1.
- With its high sensitivity and specificity, this kit is ideal for high-throughput screening in drug development, aiding researchers in identifying promising Integrin α4β7-targeted inhibitors.
- Integrin αVβ6 overexpression cell lines: These cell lines stably express the Integrin αVβ6 antigen on their surface for long-term use. They support antibody drug activity screens (cell-level binding and blocking) and evaluate CAR molecule cytotoxicity, providing essential tools for drug development and functional testing.
ACROBiosystems performs rigorous quality control on all products, verifying properties such as purity and binding activity, and provides free protocols to facilitate integrin-targeted drug development.
Image Credit: ACROBiosystems
References
- Guo, C., et al. (2024). Abstract 6349: 2MW4991, a novel ADCC-enhanced integrin αvβ8 blocker, exhibits high anti-tumor potency and was well tolerated in cynomolgus monkeys. Cancer Research, 84(6_Supplement), pp.6349–6349. https://doi.org/10.1158/1538-7445.am2024-6349.
- Lyon, R.P., et al. (2023). SGN-B6A: A New Vedotin Antibody–Drug Conjugate Directed to Integrin Beta-6 for Multiple Carcinoma Indications. Molecular Cancer Therapeutics, 22(12), pp.1444–1453. https://doi.org/10.1158/1535-7163.mct-22-0817.
- Merck. Merck Completes Acquisition of Harpoon Therapeutics, Inc. (online) Available at: https://www.merck.com/news/merck-completes-acquisition-of-harpoon-therapeutics-inc/.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.