Since the introduction of induced pluripotent stem cells (iPSCs) by Shinya Yamanaka and his team back in 2006,1,2 there has been a growing interest in harnessing the remarkable potential of these unique cells.
In culture, iPSCs can self-renew and differentiate into any cell type derived from all three germ layers: ectoderm, mesoderm, and endoderm. This breakthrough has not only deepened our understanding of the cellular mechanisms underlying pluripotency and development but has also paved the way for advanced human-specific drug screening and disease modeling.
While iPSCs are increasingly recognized for their role in producing relevant cell models in laboratories, their potential in regenerative medicine remains significant. iPSCs could serve as an "infinite" source of cells; however, the time and cost associated with their generation continue to pose challenges for many researchers.
The ongoing establishment of iPSC banks presents a valuable opportunity for researchers to access these essential cells while allowing for the standardization of iPSC quality and reliability. As a result, there is a rising demand for cell banks offering Clinical iPSC Banking for Regenerative Medicine, utilizing high-quality iPSCs, such as those derived from Laminin 521.3
Key challenges in iPSC banking include the rapid, large-scale expansion of cells, the maintenance of iPSC stemness and differentiation capability, and the preservation of genomic stability and normal karyotypes.
Laminin 521, a recombinant human basement membrane protein, provides a defined surface for feeder-free in vitro culture of various human pluripotent stem cells (PSCs).4
Research has shown that laminin can maintain normal growth characteristics and stemness across multiple PSC lines without inducing simultaneous differentiation, including embryonic stem cells (ESCs), iPSCs, and mesenchymal stem cells (MSCs).
Additionally, laminin can be highly specific to certain cell types, supporting the differentiation of stem cells into various lineages such as cardiac muscle fibers, retinal pigmented epithelial (RPE) cells, photoreceptors, dopamine (DA) neurons, and skin keratinocytes. Thus, laminin 521 has proven effective in promoting the rapid expansion of human iPSCs.
In this study, ACROBiosystems demonstrates that laminin can sustain PSC growth for over 10 passages without any indications of karyotypic abnormalities while preserving the differentiation capabilities into all three germ layer lineages.
Therefore, Laminin 521 is being proposed as an enhanced component to effectively support the clinical iPSC banking process for applications in regenerative medicine.
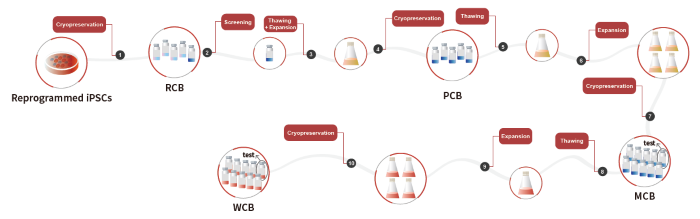
Figure 1. Workflow of clinical iPSC banking. Image Credit: ACROBiosystems
Methods and materials
Human iPSC culture with laminin 521
Laminin 521 (Cat. No. GMP-LA5H24) was applied to a 6-well TC-treated plate and incubated for 1 hour at 37 °C on day 0. Harvested iPSCs were then seeded in 2 mL of medium containing 10 µM Y27632.
On day 1, the medium was changed to exclude Y27632. The cells were incubated at 37 °C for 4–5 days with daily medium changes and were designated as P1. On days 4 to 5, iPSCs were digested using specific enzymes (e.g., Tryple, Accutase, and EDTA) and passaged up to P5 or even P10.
Cell viability assay
CellCounting-Lite™ 2.0 reagent was mixed in equal volume with the cell culture and allowed to equilibrate at room temperature. The plates were shaken and thoroughly mixed for 2–5 minutes to ensure complete cell lysis. Luminescence signals were recorded after a 10-minute stabilization period at room temperature.
Stemness maintenance assay
Cells were harvested and fixed with 4 % paraformaldehyde for 15 minutes, followed by permeabilization using 0.5 % Triton-100 for another 15 minutes. The expression of stem cell markers (OCT4, SOX2, SSEA4) was analyzed via flow cytometry.
Additionally, immunofluorescence was performed to detect the expression of stem cell markers (OCT4, NANOG, SOX2).
Karyotype analysis
iPSCs cultured for 10 passages on laminin 521 were treated with colchicine (final concentration 0.3 µg/ml) for 3 hours at 37 °C. The cells were then placed in a pre-warmed hypotonic solution for 15 minutes in a 37 °C water bath.
After fixation for 2 minutes, Giemsa solution was applied for 25 minutes for staining. Images were captured using a Nikon microscope.
Differentiation potential analysis of the three germ layers
Ectoderm differentiation
iPSCs were cultured in neural differentiation medium (DMEM/F12, B27 supplement, penicillin-streptomycin, SHH, Noggin, SB431542, dorsomorphin, LDN-193189) for 7 days, then switched to neuronal progenitor medium (DMEM/F12, B27 supplement, GlutaMAX, penicillin-streptomycin, SHH, FGF8b, dorsomorphin, LDN-193189) for 10 days, and finally, cultured in NB Maturation medium (Neurobasal, B27 supplement, GlutaMAX, penicillin-streptomycin, BDNF, GDNF, ascorbic acid, cAMP, and DAPT) for 7 additional days.
The cells were harvested, and the expression of dopaminergic neuron markers, TH-1 and MAP2, was assessed using immunofluorescence.
Endoderm differentiation
iPSCs were cultured in differentiation medium (RPMI 1640 Medium, GlutaMAX, B27 supplement, penicillin-streptomycin, Activin A) for 3 days. The cells were then harvested, and the expression of the endoderm marker, FOXA2, was evaluated by immunofluorescence.
Mesoderm differentiation
iPSCs were cultured in differentiation medium (RPMI 1640 Medium, GlutaMAX, B27 supplement, N2 supplement, penicillin-streptomycin, BMP4, CHIR99021) for 3 days. The cells were harvested, and the expression of the mesoderm marker, Brachyury, was assessed through immunofluorescence.
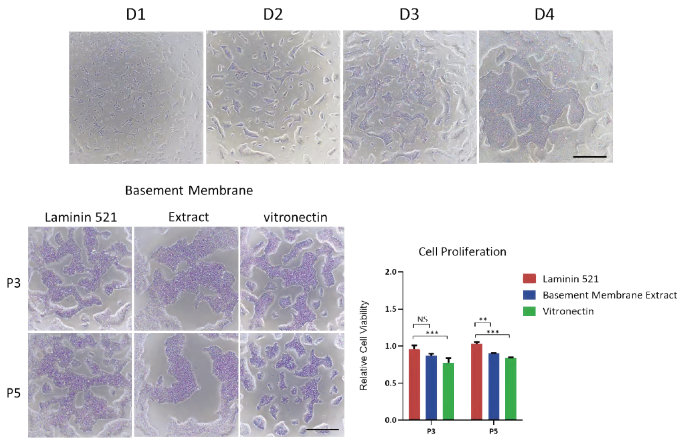
Figure 2. A) Laminin 521 effectively maintains the proliferation of human iPSC for 4 days. Scale bar, 500 μm. B) Laminin 521 has the better proliferation ability for human iPSC compared to other commercial basement membrane extract and vitronectin. Scale bar, 500 μm. Image Credit: ACROBiosystems
Results
Workflow of clinical iPSC banking
iPSCs are gaining prominence and play a crucial role in regenerative medicine. The ongoing development of iPSC banks enhances researchers' access to these essential cells while also standardizing their quality and reliability.
The cell banking process follows a three-tier management model: primary cell bank (PCB), master cell bank (MCB), and working cell bank (WCB). Cells in the PCB are expanded through thawing and amplification to form the MCB.
A similar procedure is then used to generate the WCB from the MCB. All three banks—PCB, MCB, and WCB—must be evaluated using standard indicators, including rapid expansion capability, maintenance of stemness, differentiation potential into the three lineages, and genomic stability. The workflow for clinical iPSC banking is depicted in Fig. 1.
Laminin 521 effectively promotes the rapid expansion of human iPSCs
The ability to expand iPSCs on a large scale in a short timeframe is critical for effective iPSC banking, with expansion ratios being a key consideration.
ACROBiosystems evaluated the amplification efficiency of iPSCs grown on laminin 521.
Laminin 521 (Cat. No. GMP-LA5H24) successfully maintained rapid expansion of human iPSCs, enabling cell passaging after just 4 days of culture (Fig. 2A). The proliferation capacity of iPSCs with laminin 521 exceeded that of commercially available basement membrane extracts and vitronectin protein typically used in the industry (Fig. 2B).
Moreover, laminin 521 maintained satisfactory adhesion characteristics even at low cell densities (data not shown). In conclusion, laminin 521 is effective in supporting the rapid expansion required for iPSC banking.
Laminin 521 maintains iPSC stemness after several passages
Ensuring high-quality iPSCs is essential for clinical banking and subsequent differentiation processes. The maintenance of stemness and tri-lineage differentiation ability are considered the gold standards for iPSC quality.
ACROBiosystems assessed the stemness of iPSCs cultured on laminin 521 after multiple passages. iPSCs treated with laminin 521 exhibited high expression levels of OCT4, NANOG, SOX2, and SSEA4, as shown by immunofluorescence staining and flow cytometry (Fig. 3A & Fig. 3B). This indicates that laminin 521 effectively preserves stemness across several passages.
To further evaluate the tri-lineage differentiation potential of iPSCs, a differentiation protocol was implemented and related marker gene expression was assessed through immunofluorescence staining and flow cytometry.
iPSCs cultured on laminin 521 demonstrated significant potential for ectoderm, endoderm, and mesoderm differentiation, evidenced by the expression of dopaminergic neuron markers (TH-1, MAP2), FOXA2, and Brachyury, respectively (Fig. 3C).
These findings collectively confirm that laminin 521 effectively maintains both stemness and differentiation potential of human iPSCs throughout the banking process.
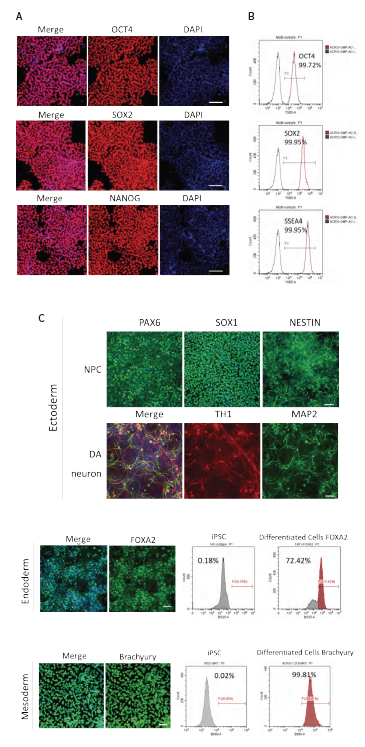
Figure 3. (A) Immunofluorescence staining of stem cell markers (OCT4, SOX2, NANOG) of iPSC coated with Laminin 521 after several passages. Scale bar, 100 μm. (B) Flow cytometry analysis of stem cell markers (OCT4, SOX2, SSAE4) of iPSC coated with Laminin 521 after several passages. (C) Immunofluorescence staining and flow cytometry analysis of iPSCs derived ectoderm (NPC: PAX6, SOX1 and NESTIN; DA neuron: TH1 and MAP2), endoderm (FOXA2), and mesoderm (Brachyury) differentiation. Scale bar, 100 μm. Image Credit: ACROBiosystems
Laminin 521 maintains normal karyotype after 10 passages
Genomic stability and chromosome integrity are vital for ensuring high-quality iPSCs during the banking process.
ACROBiosystems analyzed the karyotype of iPSCs cultured on laminin 521 after 10 passages. A normal karyotype (46, XX) was observed using the G-banding technique (Fig. 4), indicating that laminin 521 supports the genomic stability of iPSCs over an extended period.
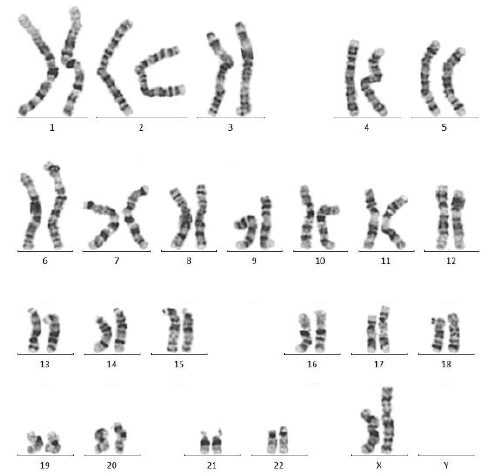
Figure 4. Karyotype analysis of iPSC with laminin 521 coating after 10 passages. Image Credit: ACROBiosystems
Conclusion
Human induced pluripotent stem cells (iPSCs) have the potential to transform personalized treatment options for a wide array of diseases and to enhance current methodologies in cell-based drug discovery and disease modeling.
Establishing iPSC banks is especially important, focusing on ensuring iPSC quality. Laminin 521 effectively promotes the rapid proliferation of human iPSCs while maintaining excellent adhesion characteristics.
Notably, after multiple passages, laminin 521 continues to uphold high levels of stemness in iPSCs and supports robust tri-lineage differentiation potential. Furthermore, these high-quality iPSCs maintained a normal karyotype even after 10 passages.
In summary, laminin 521 fulfills the requirements for iPSC banking and has the potential to accelerate large-scale iPSC manufacturing processes for regenerative medicine applications.
References
- Takahashi, K. and Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, (online) 126(4), pp.663–676. Available at: https://pubmed.ncbi.nlm.nih.gov/16904174/.
- Takahashi, K., et al. (2007). Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell, (online) 131(5), pp.861–872. Available at: https://pubmed.ncbi.nlm.nih.gov/18035408/.
- Huang, C.-Y., et al. (2019). Human iPSC banking: Barriers and Opportunities. Journal of Biomedical Science, (online) 26(1).https://doi.org/10.1186/s12929-019-0578-x.
- Yap, L., et al. (2019). Laminins in Cellular Differentiation. Trends in Cell Biology, 29(12), pp.987–1000. https://doi.org/10.1016/j.tcb.2019.10.001.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.