This article is based on a poster originally authored by Valeria Chichagova, Carol de Santis, William Atkin, Hannah Steward, and Carolina Gandara.
The prevalence of non-treatable visual impairment due to retinal degeneration is increasing, impacting millions of individuals globally.
The application of animal models in studying disease progression and drug discovery is hindered by significant structural and functional differences compared to the human retina.
Consequently, there is an urgent need for in vitro models that accurately replicate human physiology and retinal function.
The study presented here explores methods for producing and scaling retinal organoids derived from human-induced pluripotent stem cells (iPSCs) and their applicability in in vitro toxicology studies.
Methods
Retinal organoids were differentiated at scale using control human iPSCs in a 96-well plate format to facilitate high-throughput analysis.
After 150 days of differentiation, the organoids were assessed for the presence of key retinal cell types through immunofluorescence analysis.
Image Credit: Newcells Biotech
Based on existing in vivo data, the organoids' responses to agents recognized as either toxic or non-toxic to the retina were evaluated. Following a 24 or 72-hour incubation, the organoids' viability was assessed using the CellTiter-Glo® 3D ATP assay.
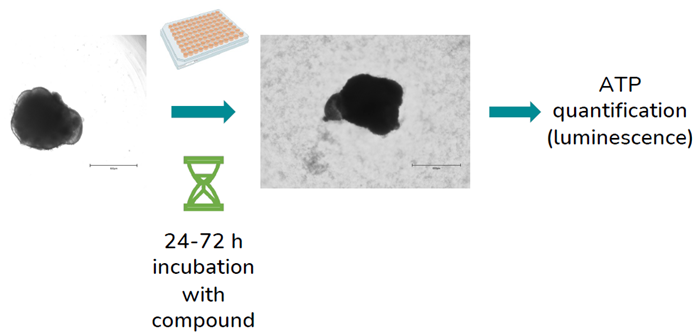
Image Credit: Newcells Biotech
Results
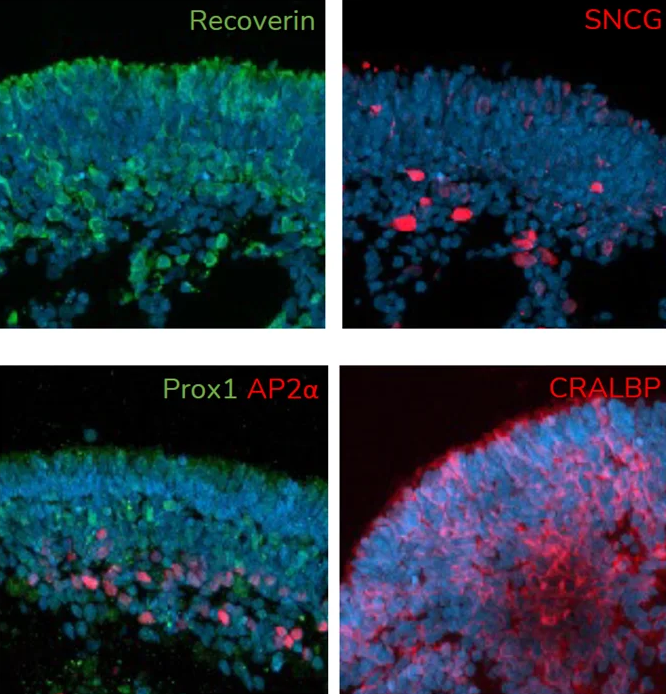
A. At day 150 of differentiation, retinal organoids contain major retinal cell types, including photoreceptors (Recoverin), retinal ganglion cells (SNCG), amacrine cells (AP2α), horizontal cells (Prox1) and Muller glia (CRALBP). Image Credit: Newcells Biotech
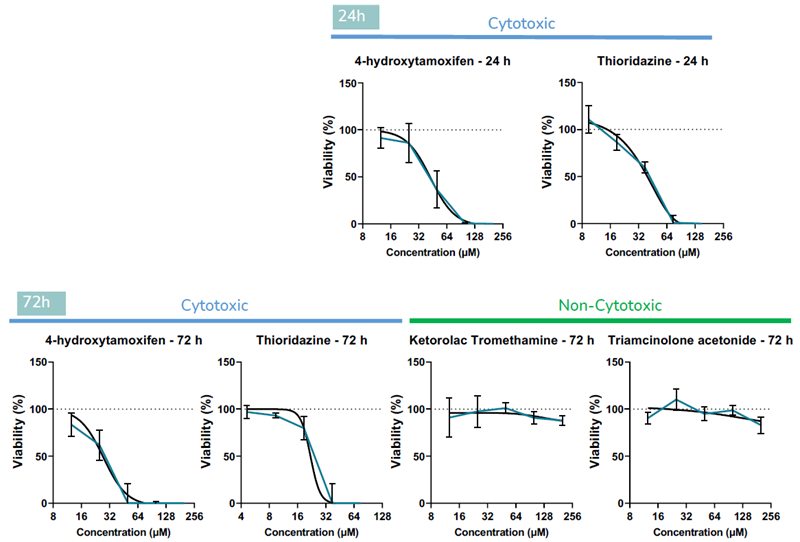
B. Retinal organoids were treated with known retinotoxins (4- hydroxytamoxifen and thioridazine), and agents known to be non-toxic to retina in vivo (ketorolac and triamcinolone acetonide) across a range of concentrations. Viability assays demonstrated that organoids respond to the compounds in a predictive doseresponse manner after 24 and 72 h of incubation. Data is presented as mean ± SD (N≥4). Image Credit: Newcells Biotech
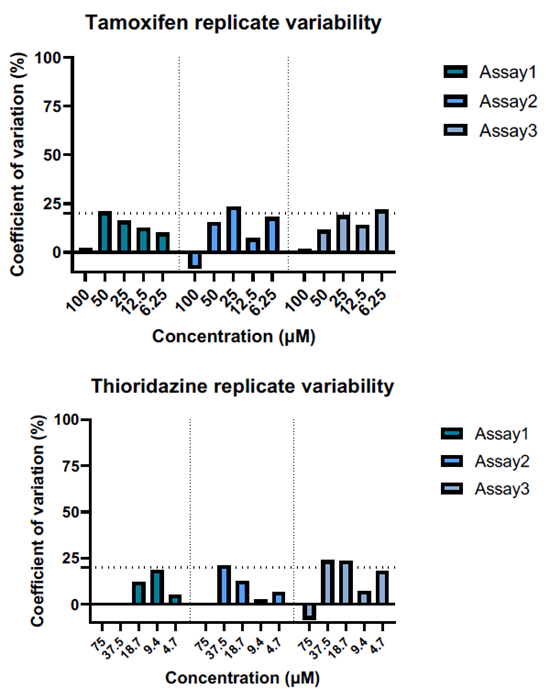
C. Organoid viability experiments were repeated across batches to demonstrate result consistency. The coefficient of variation was calculated by dividing the standard deviation (SD) by the replicate mean, and multiplied X100. Coefficient of variation % ≤ 20 % indicates good assay reproducibility. Image Credit: Newcells Biotech
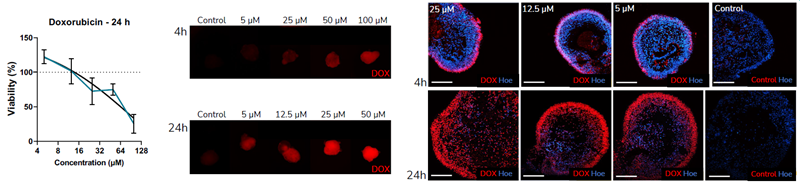
D. Retinal organoids were assessed for their suitability for studies with small molecules. In order to test whether small molecules penetrate the organoids, doxorubicin was used as a test compound. The intrinsic fluorescence of doxorubicin facilitates visualization of the drug penetration. Exposure of the iPSC-derived retinal organoids to doxorubicin reduced cell viability in a dose-dependent manner. Immunofluorescence analysis showed that doxorubicin penetrates the organoids after 4 and 24 h incubation. Image Credit: Newcells Biotech
Conclusion
Human iPSC-derived retinal organoids represent a promising in vitro tool for a variety of applications. Their utility is increasingly recognized, particularly for safety screening of new compounds.
The findings of this study demonstrated:
- Retinal organoids exhibit a dose-response relationship to compounds known to induce retinal toxicity
- Non-toxic compounds do not affect organoid viability
- Evidence for the penetration of small molecules into retinal organoids, confirming the model's suitability for this class of compounds
- Reproducible assays
The development and validation of the retinal organoid model will bridge the gap between compound screening and clinical trials, serving as a reliable model for testing the efficacy and toxicity of drugs while reducing reliance on animal models with targeted mutations as currently practiced.
Acknowledgments
Produced from materials originally authored by Valeria Chichagova from Newcells Biotech.
About Newcells Biotech
Newcells Biotech develops in vitro cell-based assays for drug and chemical discovery and development.
Using our expertise in induced pluripotent stem cells (iPSCs), cellular physiology, and organoid technology, we build models that incorporate the “best biology” for predicting in vivo behavior of new drugs.
Our experts have developed and launched assays to measure transporter function, safety, and efficacy in a range of cell and tissue types, including kidney, retina and lungs.
We have the capability to develop and implement protocols to measure cilia beat frequency and toxicity on small airway epithelial cells model, retinal toxicity and disease modelling on retinal organoids and retina epithelium, as well as drug transport in the kidney, DDI and nephrotoxicity across human and a range of preclinical species.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: May 13, 2025