Over the years, there has been an increase in scientific advancements and interests in microbial-based bioresearch. As a result, there has been growing concerns regarding the safety of employees in microbiology laboratories, as they are susceptible to infections by several pathogenic microorganisms. To help reduce these risks, strict biosafety practices have been implemented in microbiology laboratories. Biosafety involves preventative measures undertaken to eliminate pathogenic microbes and their infectious toxins.
Microbial laboratory-acquired infections are serious biohazards for laboratory workers and public health in general. Common infectious pathogenic strains dealt with frequently in microbiology laboratories include Brucella melitensis, Mycobacterium tuberculosis, and Salmonella. There are several ways in which laboratory workers can acquire infections such as through the inhalation of infectious aerosols, contact with mucous membranes, or infections through percutaneous routes.
Biosafety is implemented in microbiology laboratories through the employment of internationally recognized protocols and hazard-free laboratory procedures. In general, this includes protective barriers, correct practice of microbiological techniques and culture handling processes, proper biowaste management, appropriate and thorough sterilization/decontamination services, etc.
Image Credit: Gorodenkoff/Shutterstock.com
Laboratory Acquired Infections
Laboratory acquired infections occur due to several microbes present in microbiology laboratories including bacteria, viruses, and fungi. Exposure to pathogenic bacterial organisms seems to be the reported as the most common laboratory-acquired infection. In addition to impacting laboratory workers, these infections also pose a risk to the public, as infected laboratory employees may prove to be a transmission risk to other people.
There are several routes of infection prevalent in microbiology laboratories. For instance, infectious aerosols are typically produced by these pathogens and can be inhaled by laboratory workers. Other ways include percutaneous routes such as accidental self-inoculation, cuts, or bites. Ingestion by eating near a culture-processing workbench and direct contact are also other routes in which infections are acquired.
To implement better biosafety practices within microbiology laboratories, the World Health Organisation (WHO) has created a system where microorganisms are categorized within four different groups. The group that a microorganism is assigned to will help laboratories understand the risks associated with them, and decide which precautionary measures must be implemented to create a biosafe environment.
Within this established classification, biological agents within risk group one include microorganisms that are unlikely to cause disease to humans. Biological agents within risk group two include microorganisms that can cause disease in humans but with low chances of dissemination among laboratory workers or the public. Prophylaxis and effectual treatment modalities are available against risk group two microbes. Risk group three includes biological agents that can cause serious disease in humans with possibilities of dissemination among the community. Prophylaxis and effective treatment modalities are also available for risk three microbes. Finally, risk group four includes biological agents that cause severe illness in humans, but usually have no treatment or preventative measures available.
Brucellosis Infections Acquired in Microbiology Laboratories
As a result of unsafe practices, an outbreak of Brucella melitensis was recorded among workers within a community hospital microbiology laboratory. Brucella melitensis are a small, non-motile form of gram-negative bacteria that commonly infects a variety of animals including cattle, swine, goats, sheep, and dogs. This form of bacteria typically causes an infectious disease known as brucellosis, which remains one of the most frequent infection risks faced by microbiologists.
The outbreak caused eight employees to develop acute brucellosis, with seven of the eight workers displaying clinical illnesses ranging from non-specific flu-like symptoms to severe hepatitis. Upon further investigations, the outbreak was discovered to be due to a frozen brucella isolate from a previous hospitalized patient that had been thawed and sub-cultured without the use of a biological safety cabinet. Presumably, the bacterial isolates were transmitted via the airborne route.
Due to this outbreak, several recommendations were made to aid better biosafety practices within microbiology laboratories. In particular, it was suggested that all work on presumptive or confirmed risk group three organisms, such as Brucella melitensis, were entirely performed under biosafety hoods. Moreover, further precautions were put in place where any clinical specimens with an uncertain diagnosis were to be manipulated under biosafety hoods during the initial setup, when aerosol production is greatest, to reduce the risks of acquired infections in laboratory workers.
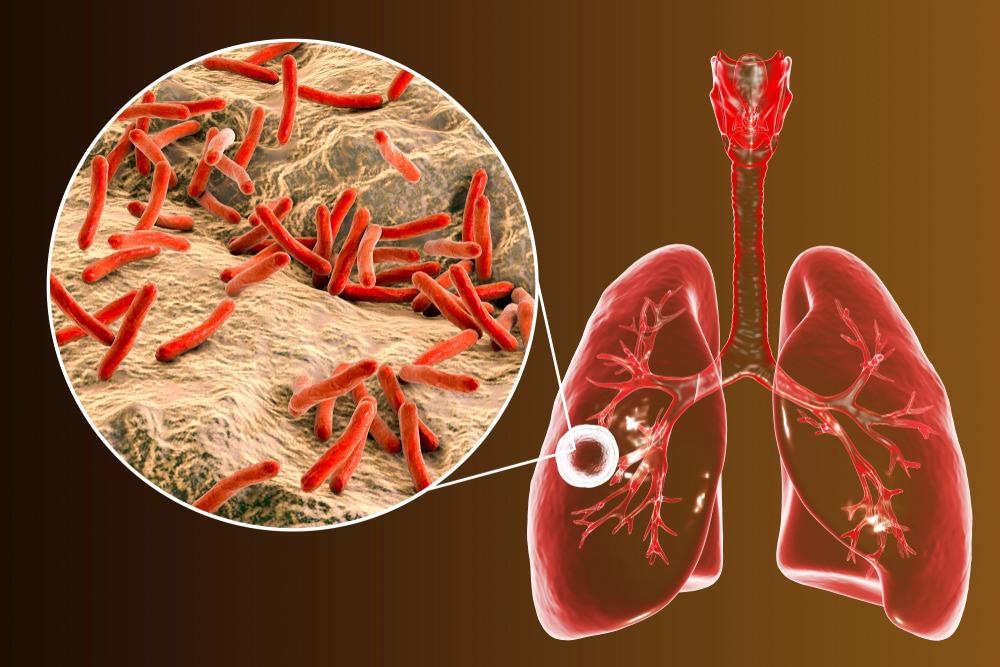
Image Credit: Kateryna Kon/Shutterstock.com
Tuberculosis infections Acquired in Microbiology Laboratories
Previous inspections have confirmed that the prevalence of pathogenic Mycobacterium tuberculosis was three to nine times higher amongst laboratory workers in comparison to the general population. Laboratory-based infections associated with Mycobacterium tuberculosis are related to infectious aerosol production by the pathogen. As a result, inadequate isolation techniques or handling high capacities of specimens, in general, were the likely cause of high infection rates.
To help reduce infection risks, annual Mantoux purified protein derivative skin tests and interferon-gamma release assays were implemented. Any laboratory workers with positive test results were expected to be further investigated for the presence of active tuberculosis by chest radiography. In conjunction with adequate and proper isolation techniques, such practices would help reduce the spread of tuberculosis infection within laboratory-based environments, thus improving biosafety.
Future Perspectives
Continuing to improve specific education and training programs for laboratory workers is necessary to help reduce infection risks in the future. With several past incidences recorded where lack of biosafety impacted laboratory workers, researchers and the scientific community can now predict future complications associated with laboratory-acquired infections and can implement such knowledge within training.
Furthermore, reporting laboratory-associated infection cases should be encouraged as good practice for all laboratory workers, since non-reported incidences have occurred in the past. Reporting these cases will continue to help supply governing bodies and regulatory authorities with useful information, allowing them to develop biosafety procedures that will prevent the release of life-threatening pathogens from microbiology laboratories.
Continue Reading: Growing Fastidious Microorganisms in the Laboratory
References:
- Byrd J, et al. (2019). Guidelines for biosafety in teaching laboratories version 2.0: A revised and updated manual for 2019. Journal of microbiology and biology education. 20: 1-3.
- Munson E, et al. (2018). Laboratory focus on improving the culture of biosafety: state-wide risk assessment of clinical laboratories that process specimens for microbiologic analysis. Journal of Clinical Microbiology. 56: e01569-17.
- Peng H, et al. (2018). Improved biosafety and biosecurity measures and/or strategies to tackle laboratory-acquired infections and related risks. International journal of environmental research and public health. 15: 1-13.
- Staszkiewicz J, et al. (1991). Outbreak of Brucella melitensis among Microbiology Laboratory Workers in a Community Hospital. Journal fo Clinical Microbiology. 29: 287-290.
Further Reading
Last Updated: Apr 13, 2022