Induced pluripotent stem cells have led to unprecedented opportunities in the pharmaceutical industry, but also in clinical and basic research. Some of the fields where induced pluripotent stem cells have created ripples are discussed below.
 metamorworks | Shutterstock
metamorworks | Shutterstock
Cell transplantation therapy
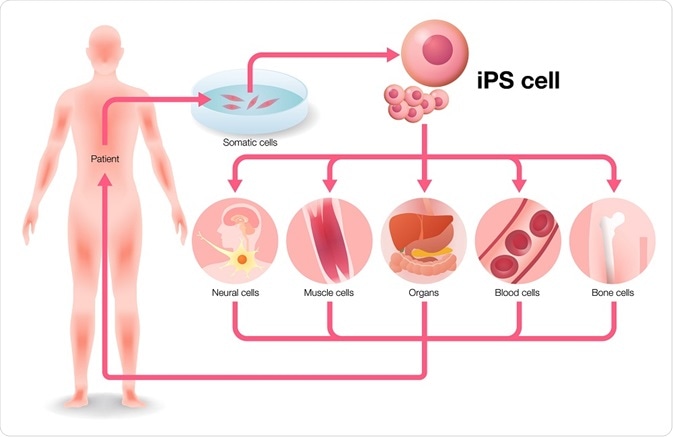
Since their discovery in 1998, embryonic stem cells were thought to the key to treat intractable diseases, including Parkinson’s disease and spinal cord injury. However, embryonic stem cells have been riddled with ethical concerns regarding their use and scientific concerns of post-transplantation rejection. In 2007, a proof-of-concept study was reported where induced pluripotent cells were used for the mouse model of sickle cell anemia.
This specific gene was corrected by using homologous recombination in the mutant iPSC cell line and subsequently transplanted into mutant mice, which led to disease cure. A major advantage of iPSC use is that donor candidates can be easily accessed. However, before creating clinical-grade iPSC clones from a donor, their health conditions and type of human leukocyte antigen (HLA) should be known. Cord blood and peripheral blood are also good sources for iPSCs.
Masayo Takahashi - 2015 Winner of Ogawa-Yamanaka Stem Cell Prize
Disease modeling and drug screening
Disease modeling and screening for drugs also constitute important applications of iPSCs. While animal models have made a tremendous contribution to our understanding of disease mechanisms, they have their limitations.
For example, several drugs showed therapeutic effects in rodent models of amyotrophic lateral sclerosis (ALS); however, all of them were ineffective in human patients. This indicates the importance of using animal cells. Currently, several patient-specific iPSC lines have been set-up and used as a model system for diseases, which will also enable the study of rare diseases.
Below is a list of other diseases in which fibroblasts have been used as source cells and lentiviral integration has been used for the generation of iPS cells.
- Huntington disease (HD)
- ALS or Lou Gehrig's disease
- Friedreich's ataxia (FRDA)
- Shwachman-Bodian-Diamond syndrome (SBDS)
- Gaucher's type III
- Becker type muscular dystrophy (BMD)
- Downs syndrome/trisomy 21
- Familial dysautonomia (FD) or Riley-Day syndrome
- Spinal muscular atrophy
- Type 1 diabetes mellitus (DM)
Basic sciences
Reprogramming technologies assist in understanding the link between epigenetic factors and cellular identity. Reprogramming also helps to assess how transcriptional and epigenetic changes are involved in cancer development.
The latter is due to the fact that reprogramming shares common events with carcinogenesis - for example, mesenchymal-epithelial transition and the involvement of c-Myc and p53. Also, reprogrammed somatic cells can help in investigating the causes of aging and the accumulation of genetic errors over time.
 FunTravel | Shutterstock
FunTravel | ShutterstockWhy are there concerns over the safety of iPS cells?
In 2007, Okita and colleagues generated induced pluripotent cell lines from mouse embryonic fibroblasts which showed embryonic stem cell-like gene expression and methylation patterns of DNA. These cell lines had similar pluripotency when compared to the embryonic stem cells; however, 20% of these chimeric mice also developed tumors due to reactivation of the c-Myc transgene.
This led to the suspicion that while induced pluripotent cells had identical properties in terms of stemness, they may not be as safe as embryonic stem cells; furthermore, the involvement of c-Myc could result in an increased risk of tumor development.
Nakagawa and colleagues tried to resolve this concern when they successfully generated induced pluripotent cells using Sox2, Klf4, and Oct3/4. The lack of c-Myc could significantly reduce the tumorigenicity risk. However, the non-usage of c-Myc also has its disadvantages, as it reduces the efficiency of induced pluripotent stem cells.
A study in 2011 showed that iPS cells could provoke an immune response when injected in mice. However, when Masumi Abe (a scientist at the National Institute of Radiological Sciences in Japan) took iPS and embryonic stem cells from mice and re-injected them, there were no differences in the immune response of the two groups, suggesting that the fears of immune response due to induced pluripotent cells may have been overestimated.
In another study, conducted at Yale University in 2012, researchers found that the reprogramming process led to fewer mutations than what was previously thought. Thus, induced pluripotent cells are a promising opportunity for therapeutic applications; however, further research and assessment is required to review all the epigenetic and genetic changes observed in induced pluripotent stem cells to determine the exact risks of this procedure.
Stem cell safety test
Further Reading
Last Updated: Jul 17, 2019