On Monday, March 25th, 2024, the U.S. FDA declined approval for Regeneron’s Odronextamab for two forms of lymphoma due to concerns over the progress of ongoing confirmatory trials. Cancer immunotherapy with CD3 bispecific antibodies (BsAbs) is a fast-developing field. As of March 2024, the FDA has approved three CD20 × CD3 BsAbs: mosunetuzumab (Lunsumio) for relapsed or refractory follicular lymphoma (R/R FL), glofitamab (Columvi) for relapsed or refractory diffuse B cell lymphoma (R/R DLBCL) or large B cell lymphoma (LBCL) with two or more prior therapies, and Epcoritamab (DuoBody®) for adult patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) after two or more systemic therapies. Progress in CD20 × CD3 bispecific antibody therapy underscores the need for ongoing research and advancement.
 Bispecific antibody. Image Credit: Sino Biological
Bispecific antibody. Image Credit: Sino Biological
Bispecific antibodies mechanism of action
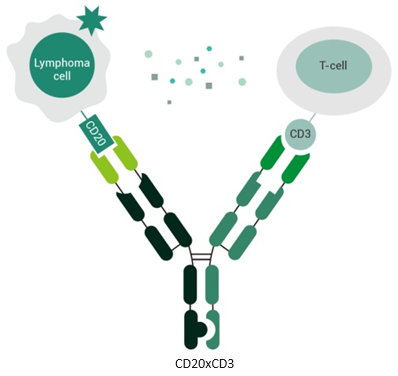
CD20 × CD3 bispecific antibodies bind to both CD20 on malignant B cells and CD3 on T cells, bringing them into close proximity, which triggers T cell-mediated killing of the targeted B cells.
- CD20 × CD3: Bridging T cells to CD20+ B-cell lymphomas
CD20 × CD3 bispecific antibodies (bsAbs) have shown effectiveness in treating various lymphomas including non-Hodgkin lymphoma (NHL), by activating T cells to attack CD20-positive B-cell lymphomas. CD20, highly expressed in B-cell lymphomas but absent in normal tissues, makes anti-CD20 drugs the main treatment for these conditions. These drugs work through various mechanisms like antibody-dependent cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and direct CD20 binding. The CD3/TCR complex facilitates the infiltration of cytotoxic T lymphocytes into cancerous cells, leading to cell death. Bispecific antibodies, targeting CD3, activate T cells to eliminate lymphoma cells by recognizing CD20. CD20 × CD3 bsAbs, engineered extensively, offer advantages like high specificity, moderate affinity, and minimal side effects. Their unique binding site allows them to effectively target CD20-positive cells, including those resistant to rituximab.
Sino Biological's offering to support CD20 × CD3 bispecific antibody research
Sino Biological partners with customers to accelerate drug discovery and development. As an international reagent supplier and service provider, Sino Biological is proud to provide a comprehensive suite of recombinant bispecific antibody production services and best-in class drug target reagents, with the ultimate goal of facilitating bispecific antibody development and screening.