Monoclonal antibodies (mAbs) are treatment options for a variety of human diseases, including cancer, cardiovascular disease, autoimmune diseases, viral infections and asthma.
Bispecific antibodies (bsAbs), unlike monospecific mAbs, have two antigen-binding sites and can thus target two different epitopes at the same time. Faricimab (Vabysmo, Roche) was approved by the FDA in January 2022 to treat wet age-related macular degeneration (AMD) and diabetic macular edema (DME).
This is the fourth bispecific antibody-drug currently on the market, besides the first bsAb Catumaxomab being withdrawn in 2017 (Table 1).
Table 1. List of Bispecific Antibody Drugs. Source: Sino Biological Inc.
| Generic Name |
Targets |
Technology |
First Approval |
Company |
Indication |
| Catumaxomab |
EPCAM/CD3 |
Quadroma |
2009
(withdrawn in 2017) |
Trion |
Malignant ascites |
| Blinatumomab |
CD19/CD3 |
BiTE |
2014 |
Amgen |
Acute lymphoblastic leukemia |
| Emicizumab |
FIX/FX |
Common LC |
2017 |
Roche |
Hemophilia A |
| Amivantamab |
EGFR/c-Met |
DuoBody |
2021 |
Genmab |
Non–small-cell lung cancer |
| Faricimab |
VEGF-A/Ang-2 |
CrossMab |
2022 |
Roche |
Diabetic macular edema, wet or neovascular, age-related macular degeneration |
Bispecific antibodies have garnered considerable attention lately, thanks to the increased interest in the field of antibodies. There are about 160 bsAbs currently in clinical trials, accounting for nearly 20% of the clinical antibody pipeline.
Design and engineering of bispecific antibodies
The quadroma method (hybrid hybridoma) was used to create bispecific antibodies initially. Only one functional bispecific antibody exists due to the random assembly of two different heavy and light chains, while the other nine variants are either monospecific or non-functional.
This results in a low yield of target bsAbs, which makes the downstream purification process more complex.
Scientists have used recombinant DNA technology to engineer bispecific antibodies to tackle the challenges of heavy and light chain association. bsAbs are divided into two categories based on their properties: IgG-like bsAbs and non-IgG-like bsAbs.
IgG-like bsAbs have a conserved immunoglobulin constant domain, so Fc-mediated effector functions like CDC and ADCC are retained. They have a long serum half-life, enhanced stability and solubility, due to their large molecular weight.
Non-IgG-like bsAbs, on the other hand, have less immunogenicity, are smaller in size and have better tissue penetration but a shorter serum half-life due to the lack of Fc fragments.
IgG-like bispecific antibodies
The interaction between the CH3 domains in IgGs allows the two heavy chains to homodimerize. Different technologies can be used to engineer the CH3 domain for Fc heterodimerization to solve the heavy chain mispairing problem. The “knobs-into-holes” (KiH) method was introduced in the 1990s and is widely used in Fc engineering.
The method entails changing a large amino acid for a small one in one antibody’s CH3 domain (the “knob”) and vice versa in the other antibody’s CH3 domain (the “hole”).
Alternative methods for producing heterodimeric bispecific antibodies, such as SEEDbody, are also used. Conversely, solving the light chain mispairing problem is also critical, and the CrossMab method is one of these technologies.
CrossMabFab, CrossMabVH-VL and CrossMabCH1-CL are the three major CrossMAb formats, as shown in Figure 1.
The bsAb light chain can be assembled correctly by changing the regions of the heavy and light chains on one side. It is frequently combined with other approaches like KiH, DEEK and ART-Ig to reduce mispairings.
Roche created a blockbuster Faricimab with dual specificities for Ang-2 and VEGFA by combining KiH and CrossMab.
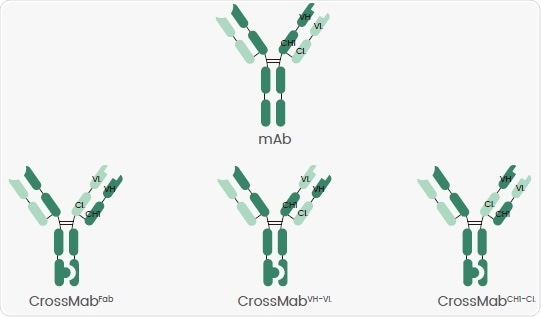
Figure 1. Different CrossMab Crossovers. Image Credit: Sino Biological Inc.
Non-IgG-like bispecific antibodies
Non-IgG-like bispecific antibodies are relatively easy to design. scFv fragments are commonly used as basic building blocks in the production of bsAbs. scFvs can form dimers, trimers, tetramers, pentamers and even higher-order oligomers when precision-engineered peptide linkers are used.
The bispecific T-cell engager (BiTE) is a type of tandem scFv that consists of two scFvs, one of which binds to CD3 on T cells and the other to a surface antigen on tumor cells, causing T cells to be redirected to destroy tumor cells.
Blinatumomab (Blincyto) was approved by the FDA for the treatment of acute lymphoblastic leukemia (ALL) using this approach. Tandem diabodies (TandAbs), dual-affinity re-targeting proteins (DARTs), dock-and-lock (DNL), single-chain diabodies and nanobodies are all common formats.
Bispecific antibody expression and production
The effectual expression and production of bispecific antibodies require the use of appropriate expression systems. Non-IgG bsAbs, such as BiTE and tandem bispecific scFv, can be expressed in E. coli, yeast, or mammalian cells, such as CHO and HEK293 cells.
E. coli is a popular choice for scFv expression since bacteria can grow quickly on low-cost media and produce large amounts of heterologous proteins.
However, the formation of intra-domain disulfide bonds, which is required for the “immunoglobulin fold” structure, is absent in expressed scFv molecules. To make soluble scFv molecules, additional protein refolding and recovery steps are generally required.
Due to their ability to perform complex post-translational modifications, mammalian cells could be used to express bispecific scFvs to overcome these issues. They are usually purified using a His-tag or protein L chromatography because they lack the Fc region.
IgG-like bsAbs, like conventional mAbs, are primarily expressed in mammalian cells, particularly CHO cells.
Bispecific antibody production, on the other hand, is more difficult due to the double number of heavy and light chain genes. Co-transfection of at least two expression plasmids into CHO cells is usually required, and the ratio of the two plasmids can affect both the quality and quantity of the expressed bsAbs.
HEK293 cells are frequently used for transient expression during the early stages of bsAb development. Transient IgG expression can be difficult to scale up, and the titers are low when compared to stable CHO cells.
Many established purification processes for conventional mAbs are compatible with bispecifics due to structural similarities between monoclonal and bispecific antibodies. A variety of purification methods are used to purify bsAbs, including charge, affinity, hydrophobicity, size and mixed-mode-based separation techniques.
Based on the expertise and experience in mammalian cell expression, Sino Biological provides fast and efficient bispecific antibody expression services to meet these manufacturing challenges.
The company delivers multiple bsAb formats, such as Diabody, BiTE, CrossMab and DVD-IgG, starting with the antibody sequence (some formats are listed in figure 2).
Sino Biological has completed many bsAb production projects with an overall success rate of >90%, with the highest yield reaching 250 mg/L.
Figure 2. Our Experience with Diverse BsAb Formats. Image Credit: The bsAb nomenclature: doi: 10.1016/j.molimm.2015.01.003.
Sino Biological highlights a recent example of bispecific antibody production. The heavy chain of the bsAb construct has a knobs-in-holes (KiH) design. The monomer purity was increased from 68.5 to 96.6% after the three-step purification process (figure 3), satisfying the final QC requirements.
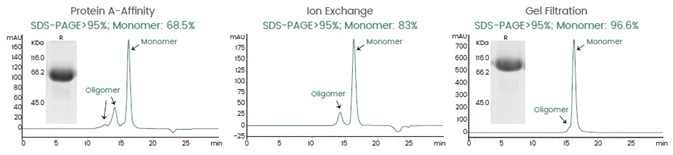 Figure 3. The Three-Step Process to Purify BsAbs. Image Credit: Sino Biological Inc.
Figure 3. The Three-Step Process to Purify BsAbs. Image Credit: Sino Biological Inc.
Concluding remarks
Diverse bispecific antibody formats are emerging to pursue optimal biological activity and clinical purposes, thanks to the advancement of recombinant DNA technology and a profound understanding of antibody engineering.
Bispecific antibodies have already shown promise in the treatment of cancer and other diseases such as Alzheimer’s disease, diabetes and ophthalmological disorders. Several bsAbs are currently in clinical development, and it is anticipated that more bsAbs will be approved for marketing in the future.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.