Autoimmune diseases result from a complex interaction of immune system imbalances, marked by a breakdown in self-tolerance and sustained inflammation. At the core of this dysregulation are cytokines—soluble signaling proteins that coordinate immune activity by facilitating communication between cells.
These cytokine networks play a dual role: they are crucial for maintaining immune stability, yet they can also drive disease processes in conditions like rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriasis, and inflammatory myopathies.
Recent findings emphasize the therapeutic value of targeting cytokines through biologic agents, Janus kinase (JAK) inhibitors, and newer approaches such as miRNA regulation and engineered cytokine therapies.
Mechanism of autoimmunity
Autoimmunity arises from intricate disruptions in immune signaling, where a combination of genetic susceptibility and environmental triggers leads to the activation of self-reactive lymphocytes, the overproduction of cytokines, and the release of autoantibodies—all contributing to tissue damage.
When resolution is possible, it relies on the re-establishment of regulatory controls, primarily through regulatory T cells (Tregs) and regulatory B cells (Bregs). These cells secrete immunosuppressive cytokines such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which play key roles in repairing tissue and dampening the inflammatory pathways that drive disease initiation and progression.
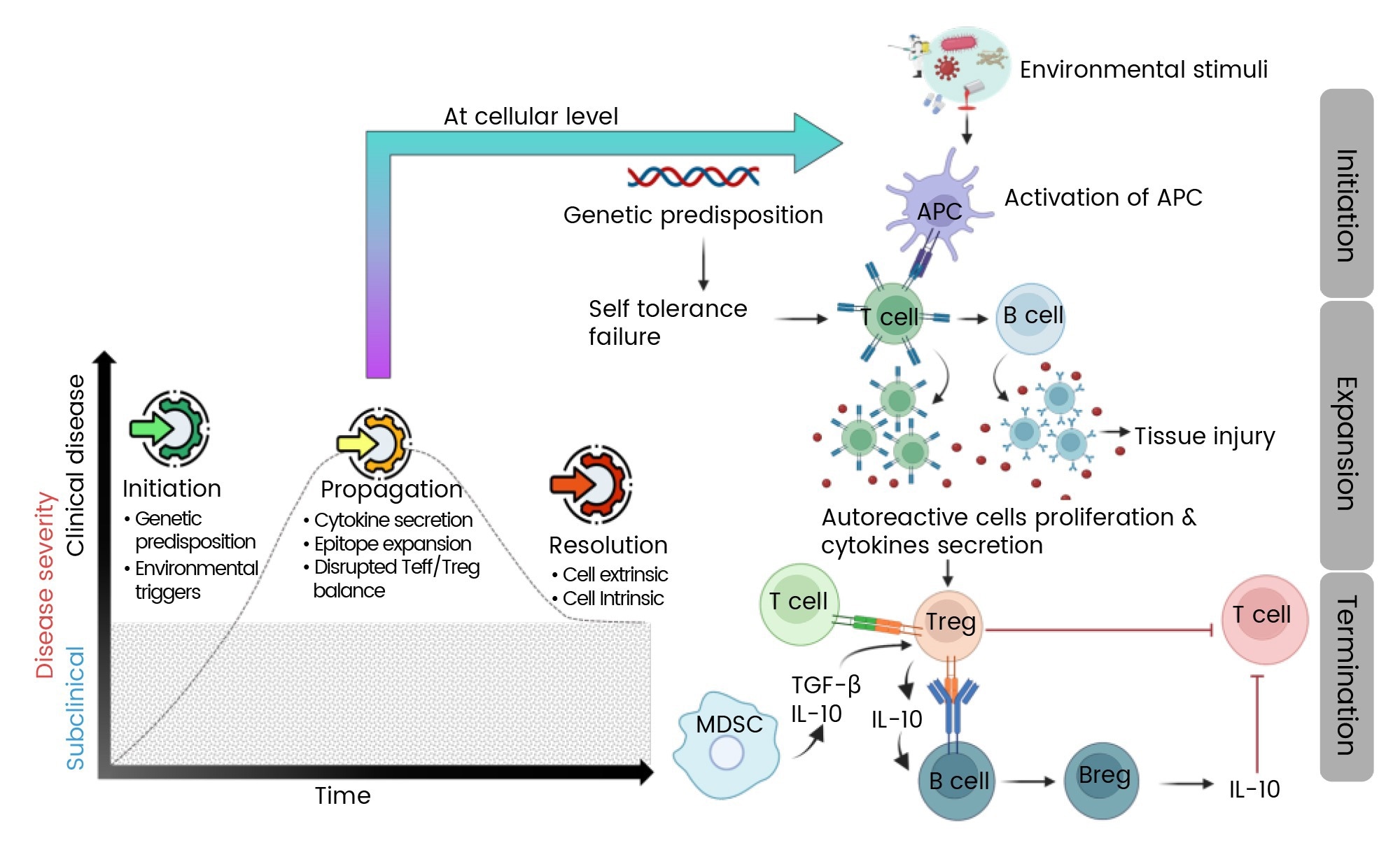
Figure 1. Mechanism of autoimmune disease progression1. Image Credit: https://doi.org/10.3390/ijms25147666
Cytokine networks in autoimmune pathogenesis
Pro-inflammatory cytokine dominance
The development of autoimmune diseases is often fueled by an overly active pro-inflammatory cytokine environment. Key players like tumor necrosis factor-alpha (TNF-α), IL-6, and IL-17 are central to the pathology of diseases such as rheumatoid arthritis (RA) and psoriasis.
Members of the IL-1 family, particularly IL-1β and IL-18, amplify tissue damage by triggering the NLRP3 inflammasome—an inflammatory mechanism also implicated in systemic lupus erythematosus (SLE).2,3
These cytokines not only sustain local inflammation but also drive systemic symptoms like fever and fatigue by acting on the hypothalamic-pituitary-adrenal axis.4
Table 1. Disease-specific cytokines. Source: Sino Biological Inc.
| Disease |
Cytokines Involved |
References |
| Rheumatoid Arthritis |
TNF-α, IL-6, GM-CSF, IL-23/IL-17 axis, IL-7 and IL-21 |
Kondo, N., et al, 2021;
Leung, S. et al, 2010 |
| Systemic Lupus Erythematosus |
IFN-α/β , IL-2, IL-1β, IL-18 and IL-12 |
Leung, S., et al, 2010;
Kotyla, P., et al, 2022 |
| Psoriasis and Psoriatic Arthritis |
IL-23/IL-17 axis (IL-17A, IL-17F), IL-22 , TNF- α and IL-6 |
Wojas-pelc, A., et al, 2006 |
| Inflammatory Myopathies |
IFN-α/β, IFN-γ, CXCL9, CXCL10, and IL-1β |
De Paepe, B., et al, 2015 |
GM-CSF: granulocyte-macrophage colony-stimulating factor
IFN-α/β: type I interferons
Regulatory cytokine deficiencies
Balancing the effects of pro-inflammatory cytokines are immunosuppressive signals from cytokines like IL-10 and TGF-β. IL-10, primarily produced by Bregs and Tregs, plays a key role in dampening immune responses by inhibiting antigen presentation and suppressing Th1 and Th17 activity.5,6
In systemic lupus erythematosus (SLE), decreased IL-10 production by Bregs has been linked to disease flares, underscoring its protective function.6 TGF-β supports peripheral tolerance by promoting Treg differentiation and inhibiting the proliferation of effector T cells.3
When these regulatory mechanisms are disrupted, as observed in rheumatoid arthritis (RA) and multiple sclerosis (MS), impaired TGF-β signaling can allow unchecked Th17 responses, fostering a pro-autoimmune environment.3,7
Cytokine imbalance and feedback loops
Autoimmune diseases are often sustained by self-amplifying cytokine feedback loops. IL-6, for example, promotes Th17 cell differentiation while suppressing Treg development, driving a cycle that perpetuates inflammation.7
In psoriasis, dendritic cell-derived IL-23 supports Th17 cell survival, which leads to the release of IL-17 and IL-22—cytokines that activate keratinocytes and stromal cells, further fueling the inflammatory response.8
These feedback mechanisms are intensified by tissue-resident cells; in rheumatoid arthritis (RA), synovial fibroblasts contribute by producing chemokines like CXCL13, which attract B cells and plasma cells and support the formation of ectopic lymphoid structures.7
Therapeutic targeting of cytokine networks
Biologic therapies
Anti-TNF-α agents: TNF inhibitors (e.g., infliximab, adalimumab) transformed RA treatment by decreasing synovitis and radiographic progression.7 Nonetheless, TNF blockade could, paradoxically, cause psoriasiform lesions in certain patients, thus undermining cytokine pleiotropy.8
IL-6 inhibition: Tocilizumab, an IL-6 receptor antagonist, improves systemic inflammation in RA and juvenile idiopathic arthritis (JIA).7 IL-6 blockade is also promising in treating neuromyelitis optica spectrum disorder (NMOSD) by decreasing the rate of relapse.
IL-17/IL-23 axis targeting: Secukinumab (anti-IL-17A) and ustekinumab (anti-IL-12/23p40) are able to induce rapid skin clearance in psoriasis by interrupting Th17 signaling.8 Brodalumab, targeting the IL-17 receptor, signals efficacy in psoriatic arthritis8.
JAK/STAT inhibition
JAK inhibitors (jakinibs) drive cytokine signaling by preventing downstream STAT phosphorylation. Tofacitinib (JAK1/3 inhibitor) and baricitinib (JAK1/2 inhibitor) are approved for RA, suppressing IFN-γ, IL-6, and GM-CSF pathways.9 In SLE, JAK inhibitors decrease IFN-α signature and improve nephritis, providing an alternative to broad immunosuppression.9
IL-2-based immunotherapies
Low-dose IL-2 broadens Tregs and restores immune tolerance in SLE and type 1 diabetes.10 Engineered IL-2 variants with enhanced Treg specificity (e.g., IL-2-anti-IL-2 complexes) showed promising results in preclinical models, though clinical trials suggest variable efficacy.10,11
miRNA modulation
miRNAs regulate cytokine production post-transcriptionally. miR-155 promotes TNF-α and IL-6 in RA synovium, while miR-146a feedback prevents NF -kB signaling.12 Antagomirs targeting miR-155 lower disease severity in experimental autoimmune encephalomyelitis (EAE), emphasizing the potential of miRNA-based therapies12.
Engineered cytokines
The objective of cytokine engineering is to enhance therapeutic specificity. PEGylated IL-10 (AM0010) lengthens half-life and suppresses colitis in preclinical models.11 Likewise, IL-4 fusion proteins bias macrophage polarization toward an anti-inflammatory phenotype, providing novel strategies for fibrosis-prone diseases like systemic sclerosis.11
Table 2. Representative FDA-approved drugs for autoimmune diseases targeting cytokines. Source: Sino Biological Inc.
| Drug Name |
Target Cytokine |
Brand Name |
FDA Approval |
Indications |
| Etanercept |
TNF-α |
Enbrel |
1998 |
Rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), ankylosing spondylitis (AS), psoriasis, psoriatic arthritis (PsA) |
| Infliximab |
TNF-α |
Remicade |
1998 |
RA, AS, psoriasis, PsA, ulcerative colitis (UC), Crohn's disease (CD) |
| Adalimumab |
TNF-α |
Humira |
2002 |
RA, JIA, AS, psoriasis, PsA, UC, CD, hidradenitis suppurativa, uveitis |
| Golimumab |
TNF-α |
Simponi |
2009 |
RA, AS, PsA, UC |
| Certolizumab |
TNF-α |
Cimzia |
2008 |
RA, AS, psoriasis, PsA, CD |
| Anakinra |
IL-1 |
Kineret |
2001 |
RA, Cryopyrin-associated periodic syndromes (CAPS) |
| Tocilizumab |
IL-6R |
Actemra |
2010 |
RA, juvenile idiopathic arthritis (JIA), adult-onset Still's disease (AOSD), giant cell arteritis, cytokine release syndrome |
| Sarilumab |
IL-6R |
Kevzara |
2017 |
RA |
| Siltuximab |
IL-6 |
Sylvant |
2014 |
Multicentric Castleman's disease |
| Satralizumab |
IL-6R |
Enspryng |
2020 |
Neuromyelitis optica spectrum disorder (NMOSD) |
| Secukinumab |
IL-17A |
Cosentyx |
2015 |
AS, psoriasis, PsA |
| Ixekizumab |
IL-17A |
Taltz |
2016 |
Psoriasis, PsA, AS |
| Brodalumab |
IL-17 receptor |
Siliq |
2017 |
Psoriasis |
| Ustekinumab |
IL-12 , IL-23 |
Stelara |
2009 |
Psoriasis, PsA, CD, UC |
| Canakinumab |
IL-1β |
Ilaris |
2009 |
CAPS, systemic juvenile idiopathic arthritis (sJIA), TRAPS, HIDS/MKD, familial Mediterranean fever (FMF) |
| Satralizumab |
IL-6R |
Enspryng |
2020 |
Neuromyelitis optica spectrum disorder (NMOSD) |
| Tofacitinib |
JAK1 , JAK3 |
Xeljanz |
2018 |
RA, PsA, UC, AS, JIA |
Summarized based on Jung S and Kim W’s paper.13
Abbreviations:
- RA: Rheumatoid Arthritis
- AS: Ankylosing Spondylitis
- PsA: Psoriatic Arthritis
- UC: Ulcerative Colitis
- CD: Crohn's Disease
- JIA: Juvenile Idiopathic Arthritis
- pJIA: Polyarticular Juvenile Idiopathic Arthritis
- FMF: Familial Mediterranean Fever
- NMOSD: Neuromyelitis Optica Spectrum Disorder
Conclusion
Advancements in cytokine biology have revealed disease-specific signatures that pave the way for precision therapies targeting critical points within cytokine networks. While progress has been significant, several challenges persist, namely cytokine redundancy, pleiotropic effects, and variability between patients.
Looking ahead, promising strategies include microbiome-based interventions, personalized cytokine profiling, and engineered biologics with improved cell-type specificity. With deeper mechanistic understanding and innovative therapeutic design, the future of autoimmune disease treatment lies in leveraging cytokine networks to reestablish immune balance.
To support focused research and therapeutic development, Sino Biological offers a broad selection of high-quality cytokine products. Each product undergoes rigorous quality control to ensure high purity, verified bioactivity, stability, and low endotoxin levels. Options are available for multiple species, including human, mouse, monkey, and rat.
Sino Biological also delivers comprehensive research solutions for nearly 50 autoimmune conditions, featuring target proteins, cytokines, kinases, and other tools for biomarker discovery. By equipping researchers with reliable, well-characterized reagents, Sino Biological contributes meaningfully to the advancement of early detection and targeted therapies in autoimmune disease research.
Featured Products for Autoimmune Research. Source: Sino Biological Inc.
References and further reading
- Farzana Yasmeen, et al. (2024). Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. International Journal of Molecular Sciences, 25(14), pp.7666–7666. https://doi.org/10.3390/ijms25147666.
- De Paepe, B. and Zschüntzsch, J. (2015). Scanning for Therapeutic Targets within the Cytokine Network of Idiopathic Inflammatory Myopathies. International Journal of Molecular Sciences, 16(8), pp.18683–18713. https://doi.org/10.3390/ijms160818683.
- Leung, S., et al. (2010). The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cellular & Molecular Immunology, 7(3), pp.182–189. https://doi.org/10.1038/cmi.2010.22.
- Biscetti, L., et al. (2021). Headache and immunological/autoimmune disorders: a comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. Journal of Neuroinflammation, 18(1). https://doi.org/10.1186/s12974-021-02229-5.
- de Gruijter, N.M., Jebson, B. and Rosser, E.C. (2022). Cytokine production by human B cells: role in health and autoimmune disease. Clinical and Experimental Immunology, [online] 210(3), pp.253–262. https://doi.org/10.1093/cei/uxac090.
- Lino, A.C., et al. (2015). Cytokine-producing B cells: a translational view on their roles in human and mouse autoimmune diseases. Immunological Reviews, 269(1), pp.130–144. https://doi.org/10.1111/imr.12374.
- Kondo, N., Kuroda, T. and Kobayashi, D. (2021). Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. International Journal of Molecular Sciences, 22(20), p.10922. https://doi.org/10.3390/ijms222010922.
- ResearchGate. (2023). (PDF) Cytokine network in psoriasis. Cross-talk between keratinocytes and cells of the skin immune system. (online) Available at: https://www.researchgate.net/publication/281604116_Cytokine_network_in_psoriasis_Cross-talk_between_keratinocytes_and_cells_of_the_skin_immune_system.
- Kotyla, P., et al. (2022). Jak Inhibitors for Treatment of Autoimmune Diseases: Lessons from Systemic Sclerosis and Systemic Lupus Erythematosus. Pharmaceuticals, 15(8), p.936. https://doi.org/10.3390/ph15080936.
- Raeber, M.E., et al. (2023). A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. eBioMedicine, (online) 90. https://doi.org/10.1016/j.ebiom.2023.104539.
- Deckers, J., et al (2023). Engineering cytokine therapeutics. Nature Reviews Bioengineering, 1(4), pp.286–303. https://doi.org/10.1038/s44222-023-00030-y.
- Salvi, V., et al. (2019). Cytokine Targeting by miRNAs in Autoimmune Diseases. Frontiers in Immunology, (online) 10. https://doi.org/10.3389/fimmu.2019.00015.
- Jung, S.M. and Kim, W.-U. (2022). Targeted Immunotherapy for Autoimmune Disease. Immune Network, 22(1). https://doi.org/10.4110/in.2022.22.e9.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.