Antibody-drug conjugates (ADCs) have revolutionized the oncology field, offering targeted cancer treatments that improve efficacy without adversely affecting healthy cells.1
ADCs’ potential extends far beyond cancer care, however, with advances in ADC technology paving the way for breakthroughs across a range of therapeutic areas from metabolic disorders to autoimmune diseases.2 This article looks at how ADCs are improving patient outcomes and redefining treatment paradigms across diverse fields of medicine.
ADCs are a pioneering class of therapeutics that combine cytotoxic drugs and monoclonal antibodies, connected via chemical linkers.
The monoclonal antibody specifically binds to antigens on diseased cells, facilitating precise drug delivery. The linker is cleaved once the monoclonal antibody has been internalized by the target cell, releasing the cytotoxic drug to apply its therapeutic effect.1,3
ADCs were originally developed as a means of treating cancer, but they have since demonstrated a unique ability to target diseases at a molecular level, offering reduced toxicity and improved precision versus traditional therapies.2
Advances in linker technology, payload engineering, and antigen discovery have seen ADCs fueling innovation across a range of therapeutic areas, including inflammatory conditions, infectious diseases, autoimmune diseases, neurological disorders, and metabolic diseases.2,3,4
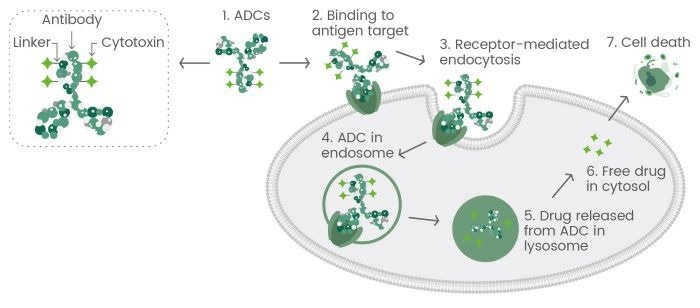
Figure 1. The general mechanism of action for antibody-drug conjugates (ADCs). Image Credit: Sino Biological Inc.
Autoimmune diseases: Precision in immune modulation
Autoimmune diseases have become a major focus for non-cancer-related ADC development. Conditions such as lupus, psoriasis, rheumatoid arthritis, ulcerative colitis, and scleroderma are typically driven by dysregulated immune responses that prompt the body to attack its own tissues.
While conventional immunosuppressive therapies are effective, these often come coupled with serious side effects due to their lack of specificity.
ADCs offer a highly targeted strategy for the treatment of autoimmune diseases such as rheumatoid arthritis (RA), however.
For instance, ABBV-3373 from AbbVie combines adalimumab (an anti-TNF monoclonal antibody) with a glucocorticoid receptor modulator (GRM), specifically delivering the GRM payload to immune cells expressing TNFα, modulating inflammatory pathways while minimizing systemic side effects.5
ADCs targeting CD6 are another promising development. These ADCs selectively eliminate pathogenic T cells implicated in autoimmune disorders such as lupus and graft-versus-host disease.6
CD45-targeted ADCs also aim to reset the immune system through the eradication of autoreactive immune cells, facilitating autologous hematopoietic stem cell transplants in conditions such as multiple sclerosis.7
ADCs also have the potential to treat inflammatory bowel diseases, targeting gut-specific inflammatory markers in order to ensure that anti-inflammatory agents are precisely delivered to the intestines.8
Combating infectious diseases
ADCs represent a promising solution to the mounting threat of antimicrobial resistance.
RG7861 is one notable example. This ADC uses a monoclonal antibody that binds to wall teichoic acid—a key bacterial structure—conjugated with a rifamycin analog. This design allows RG7861 to penetrate bacterial defenses and deliver its therapeutic payload effectively. Clinical trials have shown promising results, highlighting its potential as a next-generation antibiotic.9,10
Research is also ongoing to develop ADCs for treating HIV-1, aiming to block viral entry and replication via the linking of monoclonal antibodies able to target viral proteins such as gp41 and gp120 to small-molecule antivirals.11
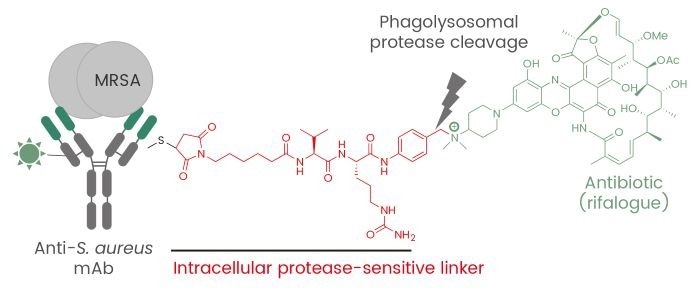
Figure 2. Antibody-antibiotic conjugate for treatment of S. aureus infections. Image Credit: DOI: 10.1038/nature16057
Fighting neurological disorders
Amyloid-beta plaques or tau protein aggregates are also known to contribute to cognitive decline in Alzheimer’s disease, meaning that ADCs designed to target these proteins could inhibit disease progression.12
ADCs also aim to modulate pathogenic immune cells or inflammatory pathways within the central nervous system, helping to treat multiple sclerosis and other autoimmune neurological conditions.
New research is also exploring ADC applications for conditions such as Huntington’s and Parkinson’s disease, potentially improving patient outcomes via targeted drug delivery. While these approaches are still experimental, the potential to address drug delivery challenges in the brain represents a major advancement in the treatment of a range of neurological disorders.2,13
Addressing metabolic disorders
ADC technology is seeing increased use in the targeting of metabolic disorders, such as atherosclerosis, diabetes, and obesity.2,14,15
Unlike traditional ADCs, metabolic applications typically involve non-cytotoxic payloads that have been specifically designed to precisely modulate biochemical pathways.
For instance, ADCs targeting lipid metabolism are currently being developed to address atherosclerosis. An LXR agonist–ADC is also being designed to deliver its payload to specific lipid pathways, helping to reduce plaque formation and modulate cholesterol levels.15,16
Table 1. Selected ADCs that have been tested for indications other than oncology. Source: Sino Biological Inc.
| Indications |
ADCs |
Antibody |
Linkers |
Payloads |
| Inflammation |
Dexa-AbhEsel |
Murine anti-E-selectin mAb (H18/7) |
Succinate |
Dexamethasone |
| Autoimmune models |
Anti-CD74-flu449 |
Human anti-CD74 mAb |
Pyrophosphate acetal |
Fluticasone propionate |
| Atherosclerosis |
Anti-CD1la
LXR agonist |
Humanized anti-CD11 mAb |
PEG4-Phe-Lys |
Amino acid para-acetylphenylalanine |
Muscular
diseases |
Anti-CD71 siRNA |
Murine anti-CD71 |
mAb Maleimide |
siRNA |
Systemic
sclerosis |
ADCETRIS |
Chimeric anti-CD30 mAb |
Val-Cit |
MMAE |
| Non-alcoholic fatty liver disease |
Anti-CD163-IgG-Dex |
Anti-CD163 mAb |
Hemisuccinate |
Dexamethasone |
| Lung infection |
VSX-D297 antimicrobial
antibody conjugate |
VSX |
Enzymatically coupling
with Sortase A |
Antimicrobial
peptides |
Conclusion and future outlook
ADCs have progressed from being principally oncology-focused to being versatile tools with the potential to revolutionize a range of therapeutic areas. ADCs’ capacity to combine precision targeting with potent therapies provides a viable pathway to the treatment of a range of complex diseases, from autoimmune disorders to metabolic and neurological conditions, as well as addressing the increasingly prevalent issue of antimicrobial resistance.
Future developments in antibody design, linker technology, and payload engineering are expected to further expand the applications of ADCs, while the integration of ADCs with emerging fields like nanotechnology, gene therapy, and personalized medicine has the potential to unlock new possibilities.
Clinical research continues to validate their safety and efficacy, meaning that ADCs are set to become central to modern therapeutics, improving patient outcomes and addressing unmet medical needs.1–4,17–21
Sino Biological's support for ADC development
Sino Biological is supporting pioneering ADC research with its diverse portfolio of products and services. The company’s end-to-end solutions and seamless client support drive every stage of the ADC development process, from early discovery through clinical development.
Sino Biological’s strong focus on recombinant protein production sees the company offer a diverse range of high-quality ADC target proteins available in multiple formats and species.
Its catalog includes well-known targets such as Nectin-4, EGFR, HER-2, TROP-2, CD19, and BCMA, along with emerging targets like GFRA1, EphA3, and CLEC7A.
The company also provides comprehensive solutions for research into the treatment of autoimmune diseases, including target proteins for nearly 50 diseases. These reagents are designed to support the application of innovative ADCs in the treatment of autoimmune diseases.
References and further reading
- Tsuchikama, K., et al. (2024). Exploring the next generation of antibody–drug conjugates. Nature Reviews Clinical Oncology, (online) pp.1–21. https://doi.org/10.1038/s41571-023-00850-2.
- Lal Bahadur Pal, Bule, P., Khan, W. and Naveen Chella (2023). An Overview of the Development and Preclinical Evaluation of Antibody–Drug Conjugates for Non-Oncological Applications. Pharmaceutics, 15(7), pp.1807–1807. https://doi.org/10.3390/pharmaceutics15071807.
- Conilh, L., et al. (2023). Payload diversification: a key step in the development of antibody–drug conjugates. Journal of Hematology & Oncology, 16(1). https://doi.org/10.1186/s13045-022-01397-y.
- Dumontet, C., et al. (2023). Antibody–drug conjugates come of age in oncology. Nature Reviews Drug Discovery, (online) 22(8), pp.641–661. https://doi.org/10.1038/s41573-023-00709-2.
- McPherson, M.J., et al. (2024). An anti-TNF-glucocorticoid receptor modulator antibody-drug conjugate is efficacious against immune-mediated inflammatory diseases. Science translational medicine, (online) 16(739), p.eadd8936. https://doi.org/10.1126/scitranslmed.add8936.
- Zhang, L., et al. (2023). A CD6-targeted antibody-drug conjugate as a potential therapy for T cell-mediated disorders. JCI insight, (online) 8(23), p.e172914. https://doi.org/10.1172/jci.insight.172914.
- Brandish, P.E., et al. (2018). Development of Anti-CD74 Antibody–Drug Conjugates to Target Glucocorticoids to Immune Cells. Bioconjugate Chemistry, 29(7), pp.2357–2369. https://doi.org/10.1021/acs.bioconjchem.8b00312.
- Rimola, J., et al. (2024). ADC Values for Detecting Bowel Inflammation and Biologic Therapy Response in Patients With Crohn Disease: A Post Hoc Prospective Trial Analysis. AJR. American journal of roentgenology, (online) 222(1), p.e2329639. https://doi.org/10.2214/AJR.23.29639.
- Lehar, S.M., et al. (2015). Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature, (online) 527(7578), pp.323–328. https://doi.org/10.1038/nature16057.
- ADC Review. (2017). DSTA4637S (Anti-S. aureus TAC; RG7861)» ADC Review. (online) Available at: https://www.adcreview.com/drugmap/dsta4637s/.
- Umotoy, J.C. and de Taeye, S.W. (2021). Antibody Conjugates for Targeted Therapy Against HIV-1 as an Emerging Tool for HIV-1 Cure. Frontiers in Immunology, 12. https://doi.org/10.3389/fimmu.2021.708806.
- Punyakoti, P., et al. (2023). Postulating the possible cellular signalling mechanisms of antibody drug conjugates in Alzheimer’s disease. Cellular Signalling, (online) 102, p.110539. https://doi.org/10.1016/j.cellsig.2022.110539.
- Drug Target Review. (2024). Exploring the potential of ADCs beyond oncology. (online) Available at: https://www.drugtargetreview.com/article/151932/exploring-the-potential-of-adcs-beyond-oncology/.
- Xue, D., et al. (2019). An anti-CD103 antibody-drug conjugate prolongs the survival of pancreatic islet allografts in mice. Cell death & disease, (online) 10(10), p.735. https://doi.org/10.1038/s41419-019-1980-8.
- Liu, Y.-B., et al. (2024). A sterol analog inhibits hedgehog pathway by blocking cholesterylation of smoothened. Cell chemical biology, (online) 31(7), pp.1264-1276.e7. https://doi.org/10.1016/j.chembiol.2024.02.002.
- AYYAPPAN, J.P., PAUL, A. and GOO, Y.-H. (2016). Lipid droplet-associated proteins in atherosclerosis (Review). Molecular Medicine Reports, (online) 13(6), pp.4527–4534. https://doi.org/10.3892/mmr.2016.5099.
- Valsasina, B., et al. (2024). Present Scenario and Future Landscape of Payloads for ADCs: Focus on DNA-Interacting Agents. Pharmaceuticals, (online) 17(10), pp.1338–1338. https://doi.org/10.3390/ph17101338.
- Ruiz, R. and Kirk, A.D. (2015). Long-Term Toxicity of Immunosuppressive Therapy. Transplantation of the Liver, pp.1354–1363. https://doi.org/10.1016/b978-1-4557-0268-8.00097-x.
- Gu, Y., Wang, Z. and Wang, Y. (2024). Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B. (online) https://doi.org/10.1016/j.apsb.2024.01.009.
- Beck, A., et al. (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nature Reviews Drug Discovery, 16(5), pp.315–337. https://doi.org/10.1038/nrd.2016.268.
- Carter, P.J. and Lazar, G.A. (2017). Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nature Reviews Drug Discovery, 17(3), pp.197–223. https://doi.org/10.1038/nrd.2017.227.
Acknowledgments
Produced from materials originally authored by Sino Biological.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.