Autoimmune diseases are all characterized by the immune system's misrecognition of self-antigens, despite representing a diverse array of disorders affecting the entire body or specific organs.
These conditions stem from a combination of environmental and genetic factors, prompting the immune system to fail to properly distinguish between the body's own structures and foreign pathogens, prompting sustained attacks on healthy tissues, organs, and cells.1 This pathological process results in progressive tissue damage, chronic inflammation, fibrosis, and, ultimately, organ dysfunction.
Researchers have identified approximately 150 autoimmune diseases thus far, with several validated drug targets currently in approved therapies (Table 1).
Common autoimmune diseases include multiple sclerosis (MS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and type 1 diabetes (T1DM).2,3,4
Table 1. Approved Therapies for Autoimmune Disease and Their Targets. Source: Sino Biological Inc.
| Target |
Drug Name |
Indications |
Approved Year |
| FCRN/FCGRT |
Rozanolixizumab |
Myasthenia Gravis |
2023/6/26 |
| CD20 |
Ublituximab |
Multiple Sclerosis Relapse |
2022/12/28 |
| CD3 |
Teplizumab |
Type 1 Diabetes |
2022/11/17 |
| IL1RL2 |
Spesolimab |
Generalized Pustular Psoriasis |
2022/9/1 |
| IFNAR1 |
Anifrolumab-FNIA |
Systemic Lupus Erythematosus |
2021/7/30 |
| IGF1R |
Teprotumumab-TRBW |
Graves Ophthalmopathy |
2020/1/21 |
| CD19 |
Inebilizumab-cdon |
Neuromyelitis Optica |
2020/6/11 |
| CD20 |
Ocrelizumab |
Multiple Sclerosis |
2017/3/28 |
Autoimmune diseases share the same core pathology despite their differing clinical manifestations: dysregulated B and T cell activity.
B cells contribute to disease progression by secreting pro-inflammatory cytokines, producing autoantibodies, and functioning as antigen-presenting cells (APCs) able to activate the immune system’s other components.5
CD4⁺ T lymphocytes coordinate immune responses by activating various immune cell populations and secreting cytokines. Autoreactive T cells are generated when CD4⁺ T lymphocytes are dysregulated, however, leading to tissue damage and chronic inflammation (Figure 1).6
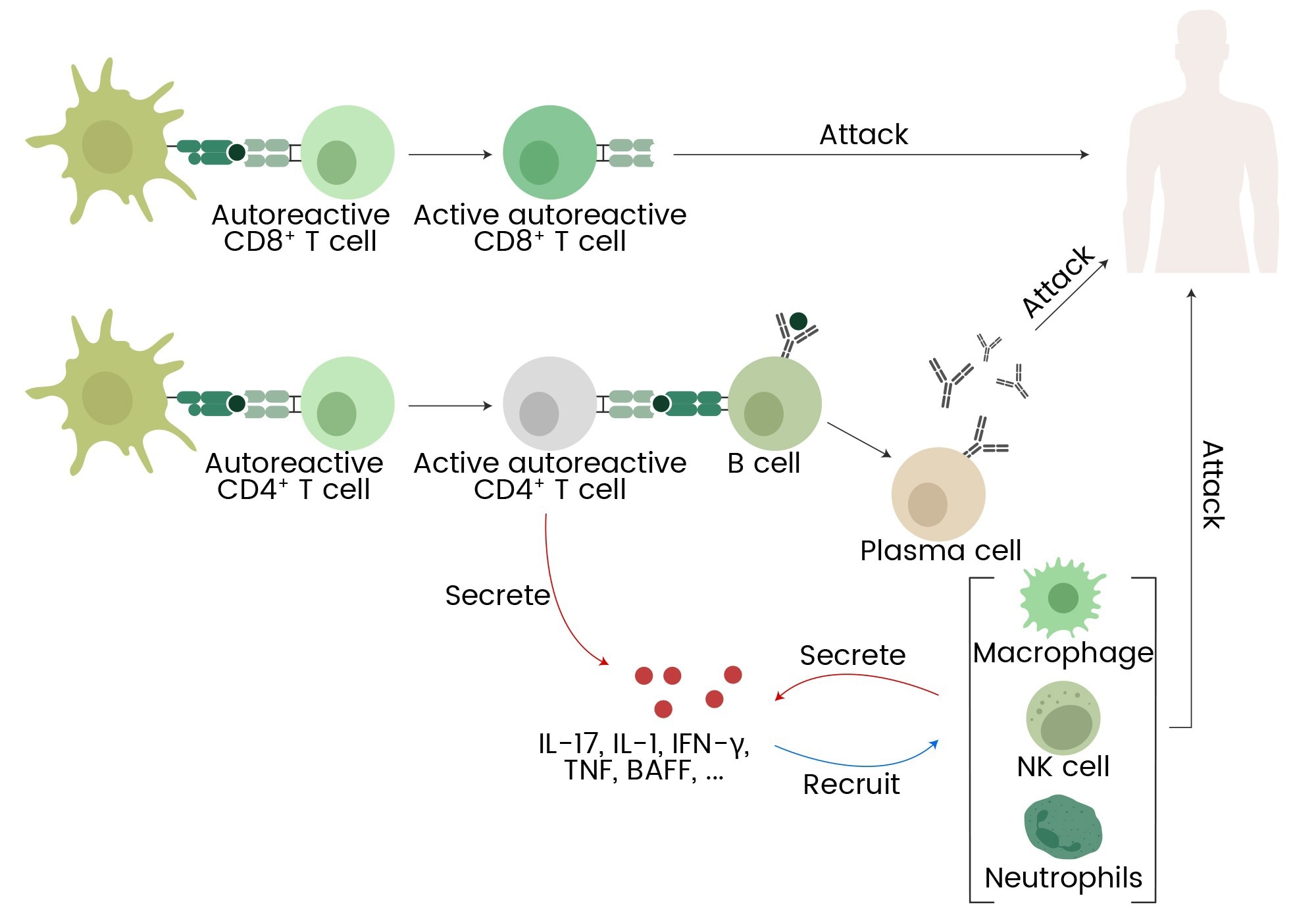
Figure 1. Autoreactive T cells, B cells, macrophages, NK cells, neutrophils, and cytokines (IL-17, IL-1, IFN-γ, TNF, BAFF) contribute to autoimmune responses and inflammation. 7 Image Credit: Sino Biological Inc.
Autoimmune diseases are becoming increasingly complex and widespread, representing a major challenge for the pharmaceutical industry and healthcare providers.
Current treatments typically lack specificity, targeting the immune system more broadly and triggering side effects, including allergic reactions and increased infection risk.8 Promising strategies with higher target specificity are now under investigation, including chimeric antigen receptor T (CAR-T) cell therapy and antibody-drug conjugates (ADC).6,9
CAR-T cell therapy: A targeted approach for autoimmune diseases
CAR-T therapy was initially developed as a treatment for cancer, but it is now being explored for autoimmune diseases. Many autoimmune diseases arise from autoreactive B and T cells, meaning that the selective elimination of these cells represents a novel therapeutic strategy.
Current research is focused on engineering CAR-T cells able to recognize and remove cells expressing certain surface markers. For example, targets like CD19, CD20, CD38, and BCMA have been investigated in B cell–mediated autoimmune disorders (Table 2), while CD7 and CD70 are being studied in T cell–driven diseases.4
CD19 appears to be the most promising and thoroughly investigated CAR-T therapy target, with clinical trials studying the use of CD19 CAR-T cells for severe SLE, MS, lupus nephritis (LN), and systemic sclerosis (SSc) demonstrating effective disease control while maintaining favorable safety profiles.10,11
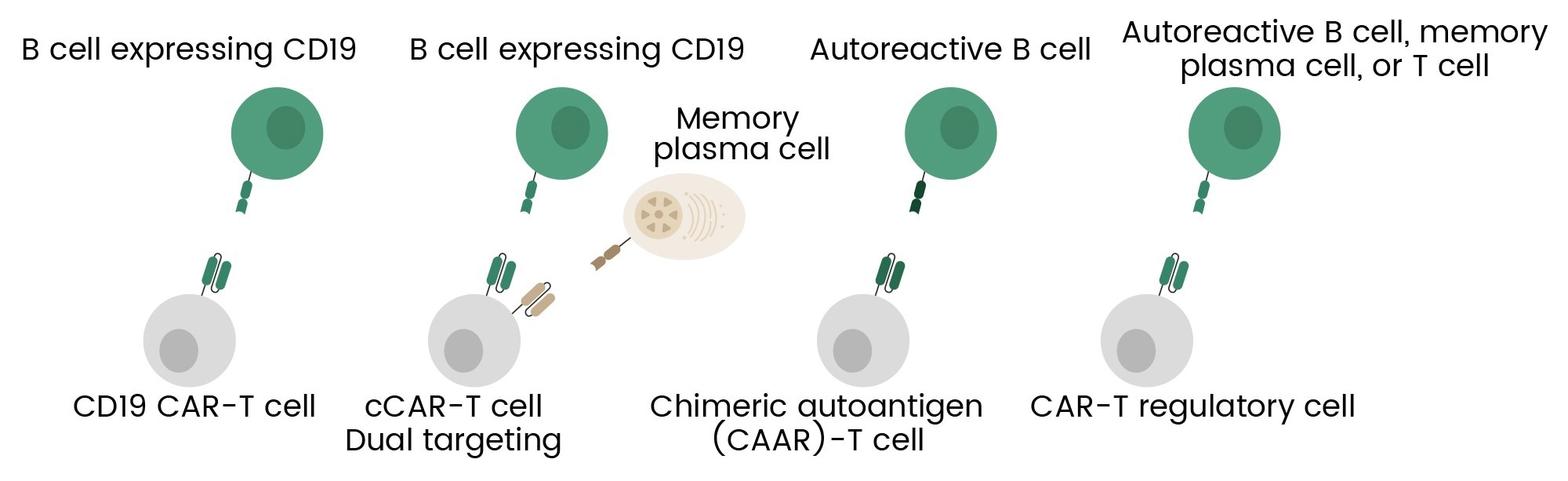
Figure 2. Overview of CAR-T cell engineering strategies for autoimmune diseases, including broad B cell targeting, dual targeting, selective depletion of autoreactive B cells, and T regulatory cell engineering.6 Image Credit: Sino Biological Inc.
For example, a recent case of severe SLE was treated with autologous CD19 CAR-T cells. This case demonstrated full and sustained depletion of circulating B cells, resulting in the disappearance of dsDNA autoantibodies. No adverse events were observed during the treatment.10
These findings are supported by a separate study involving five patients with SLE treated with CD19 CAR-T cells. In this instance, every patient achieved sustained clinical remission one year following treatment. It was also observed that naïve B cells began to re-emerge three months post-therapy, with no recurrence of SLE symptoms.
These findings suggest an effective reset of the patient’s immune system, effectively promoting long-term disease control.11
Table 2. Representative clinical trials for CAR-T cell therapy in autoimmune diseases.11 Source: Sino Biological Inc.
| Target |
Condition |
NTC |
Phase |
Sponsor |
| CD19 |
Severe, refractory systemic lupus erythematosus |
NCT05869955 |
Phase 1 |
Bristol-Myers Squibb |
| CD19 |
Systemic lupus erythematosus |
NCT05765006 |
Phase 1 |
Shanghai Ming Ju Biotechnology Co., Ltd. |
| CD19 |
Refractory lupus nephritis |
NCT05938725 |
Phase 1 |
Kyvema Therapeutics |
| CD19 |
Systemic lupus erythematosus/lupus nephritis |
NCT05798117 |
Phase 1 |
Novartis Pharmaceuticals |
| CD19 |
Refractory/Moderate-to-severe systemic lupus erythematosus |
NCT06106096 |
Phase 1/2 |
Wuhan Union Hospital, China |
CD19/
BCMA |
Relapsed/refractory systemic lupus erythematosus |
NCT05474885 |
Phase 1 |
iCell Gene Therapeutics |
CD19/
CD20 |
Refractory systemic lupus erythematosus |
NCT06153095 |
Phase 1 |
ImmPACT Bio |
| CD19 |
Relapsing/Progressive multiple sclerosis |
NCT06222001 |
Phase 1 |
Juno Therapeutics |
| CD19 |
Refractory myasthenia gravis |
NCT05828225 |
Phase 2 |
Zhejiang University |
| BCMA |
Generalized myasthenia gravis |
NCT04146051 |
Phase 1 |
Cartesian Therapeutics |
There are still a number of challenges associated with the application of CAR-T therapy to other autoimmune diseases, despite promising results in the treatment of SLE. For example, autoantibodies produced by plasmablasts or plasma cells lack CD19 expression in some instances, allowing them to evade CD19-targeted therapies.12
Additional research is required in order to refine and expand CAR-T cell strategies, adapting these therapies to the specific immunopathology of different autoimmune diseases.
ADC: Leveraging cancer therapy success for autoimmune treatment
Antibody-drug conjugates (ADCs) combine the potent cytotoxicity of cytotoxic payloads with the precision of monoclonal antibodies. Their use is well-established in cancer therapies, but ADCs now show promise for autoimmune diseases.
Antibody-based treatments like the anti-TNF monoclonal antibody are limited in the treatment of conditions like rheumatoid arthritis (RA). ADCs offer a more targeted approach, however, sparing protective immunity while selectively eliminating pathogenic immune cell subsets like autoreactive T or B cells.
A number of studies have shown promising results with ADCs targeting TNF,13 CD74,14 CD30,15 CD163,16 and CD6.17 ADCs can be leveraged to deliver immunomodulatory or cytotoxic payloads directly to pathogenic cells, reducing off-target toxicity, improving therapeutic precision, and overcoming the limitations of systemic immunosuppression in autoimmune conditions.
For example, the TNF receptor family member D30, is a potential therapeutic target due to its tendency to be elevated in RA serum and joint fluid.
Brentuximab vedotin (BV) is a CD30-targeting antibody-drug conjugate (ADC) combining brentuximab with antimitotic MMAE, and BV is currently being evaluated as a potential treatment for RA.
BV was found to significantly reduce the severity of arthritis in CAIA mouse models when applied at a higher dose (70 mg/kg), while the lower dose (30 mg/kg), equivalent to the human clinical dose, showed no significant effect.16
ADCs have shown promise for autoimmune diseases, but there are several challenges associated with their adaptation. For example, autoimmune targets such as CD30 may be expressed on both protective and pathogenic immune cell populations, increasing the chances of inadvertent immunosuppression.
ADC designs should also improve safety profiles, particularly in terms of linker stability. This is key to preventing off-target payload release and long-term immune cell depletion.
Additional research is required in order to refine target selection and optimize payload efficacy, enabling next-generation ADCs to offer the tunable cytotoxicity required for continued autoimmune disease therapy.
Conclusion and future outlook
Precision-targeted therapies are revolutionizing autoimmune disease management, addressing the many challenges associated with traditional immunosuppressants.
CAR-T cell therapy represents a promising approach due to its capacity to selectively eliminate autoreactive B and T cells, with CD19-targeted therapies shown to prompt sustained disease remission in conditions such as systemic lupus erythematosus (SLE).
ADCs leverage antibody specificity in order to deliver cytotoxic payloads, refining immune modulation strategies designed to treat autoimmune diseases.
Optimizing these therapies for broader applications will be critical as research advances, with future investigations focused on adapting ADC payloads for autoimmune specificity, the refinement of CAR-T targets beyond CD19, and mitigating off-target effects. It is anticipated that continued exploration of immunotherapy will lead to the development of more precise, long-lasting treatments.18,19
Sino Biological's autoimmune disease research portfolio
Sino Biological offers a comprehensive portfolio of autoimmune disease research solutions, including reagents for almost 50 diseases. The company’s portfolio features target proteins, cytokines, and kinases, alongside a range of biomarker research tools.
Sino Biological supports early detection and the development of targeted therapies through the delivery of high-quality reagents for biomarker analysis and drug discovery.
The company also provides CAR-T therapy development solutions and ADC development solutions. All of its autoimmune disease research solutions are based on the provision of high-quality reagents and ongoing technical support that is ideally suited to serving life science research institutions and global drug R&D companies.
References and further reading
- Davidson, A. and Diamond, B. (2001). Autoimmune Diseases. New England Journal of Medicine, 345(5), pp.340–350. https://doi.org/10.1056/nejm200108023450506.
- Georg Schett, Mackensen, A. and Dimitrios Mougiakakos (2023). CAR T-cell therapy in autoimmune diseases. The Lancet, 402(10416). https://doi.org/10.1016/s0140-6736(23)01126-1.
- Pisetsky, D.S. (2023). Pathogenesis of Autoimmune Disease. Nature Reviews Nephrology, (online) 19(8), pp.1–16. https://doi.org/10.1038/s41581-023-00720-1.
- Li, Y.-R., et al. (2024). Frontiers in CAR-T cell therapy for autoimmune diseases. Trends in Pharmacological Sciences, 45(9), pp.839–857. https://doi.org/10.1016/j.tips.2024.07.005.
- Rubin, S.J.S., Bloom, M.S. and Robinson, W.H. (2019). B cell checkpoints in autoimmune rheumatic diseases. Nature Reviews Rheumatology, 15(5), pp.303–315. https://doi.org/10.1038/s41584-019-0211-0.
- Vukovic, J., et al. (2024). CAR-engineered T cell therapy as an emerging strategy for treating autoimmune diseases. Frontiers in medicine, (online) 11, p.1447147.https://doi.org/10.3389/fmed.2024.1447147.
- Song, Y., Li, J. and Wu, Y. (2024). Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders. Signal Transduction and Targeted Therapy, (online) 9(1). https://doi.org/10.1038/s41392-024-01952-8.
- Fugger, L., Jensen, L.T. and Rossjohn, J. (2020). Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell, 181(1), pp.63–80. https://doi.org/10.1016/j.cell.2020.03.007.
- Dixit, T., Vaidya, A. and Ravindran, S. (2024). Therapeutic potential of antibody-drug conjugates possessing bifunctional anti-inflammatory action in the pathogenies of rheumatoid arthritis. Arthritis Research & Therapy, 26(1). https://doi.org/10.1186/s13075-024-03452-0.
- Mougiakakos, D., et al. (2021). CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. New England Journal of Medicine, 385(6), pp.567–569. https://doi.org/10.1056/nejmc2107725.
- Mackensen, A., et al. (2022). Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nature Medicine, (online) 28, pp.1–9. https://doi.org/10.1038/s41591-022-02017-5.
- Rampotas, A., et al. (2024). CAR-T cell therapy embarks on autoimmune disease. Bone Marrow Transplantation. (online) https://doi.org/10.1038/s41409-024-02429-6.
- Buttgereit, F., et al. (2023). Efficacy and Safety of ABBV‐3373, a Novel Anti–Tumor Necrosis Factor Glucocorticoid Receptor Modulator Antibody–Drug Conjugate, in Adults with Moderate‐to‐Severe Rheumatoid Arthritis Despite Methotrexate Therapy: A Randomized, Double‐Blind, Active‐Controlled Proof‐of‐Concept Phase IIa Trial. Arthritis & rheumatology, 75(6), pp.879–889. https://doi.org/10.1002/art.42415.
- Brandish, P.E., et al. (2018). Development of Anti-CD74 Antibody–Drug Conjugates to Target Glucocorticoids to Immune Cells. Bioconjugate Chemistry, 29(7), pp.2357–2369. https://doi.org/10.1021/acs.bioconjchem.8b00312.
- M. Matsuhashi, Nishida, K., et al. (2020). SAT0010 ANTI-CD30 IMMUNOTHERAPY AMELIORATES BONE AND CARTILAGE DESTRUCTION IN EXPERIMENTAL MODEL OF RHEUMATOID ARTHRITIS IN MICE. Annals of the Rheumatic Diseases, 79(Suppl 1), pp.935.1-935. https://doi.org/10.1136/annrheumdis-2020-eular.1039.
- Lee, H., et al. (2017). Tocilizumab-Alendronate Conjugate for Treatment of Rheumatoid Arthritis. Bioconjugate chemistry, (online) 28(4), pp.1084–1092. https://doi.org/10.1021/acs.bioconjchem.7b00008.
- Zhang, L., et al. (2023). A CD6-targeted antibody-drug conjugate as a potential therapy for T cell-mediated disorders. JCI insight, (online) 8(23), p.e172914. https://doi.org/10.1172/jci.insight.172914.
- Hartmann, J., et al. (2017). Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Molecular Medicine, 9(9), pp.1183–1197. https://doi.org/10.15252/emmm.201607485.
- J. Joseph Melenhorst, Chen, G.M., et al. (2022). Author Correction: Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature, 612(7941), pp.E22–E22. https://doi.org/10.1038/s41586-022-05376-8.
Acknowledgments
Produced from materials originally authored by Sino Biological
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.