Since the FDA first approved CAR-T cell therapy in 2017, eight CAR-T drugs have emerged, six of which have been fully approved by the FDA. This groundbreaking cell therapy has brought about a series of innovations relating to the treatment of malignant hematological tumors, thus generating an increased interest across R&D circles in both academia and industry and the development of effective cancer treatments.
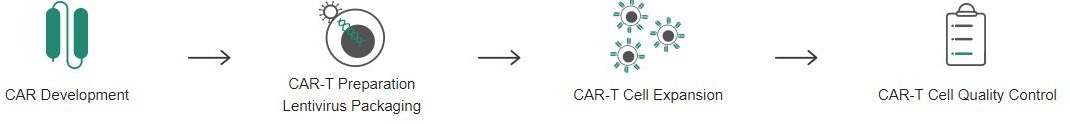
Image Credit: Sino Biological Inc.
Sino Biological is a leading supplier of bioreagents and CRO services for the biopharmaceutical field and offers a range of comprehensive solutions for CAR-T cell therapy development. Sino’s reagents and services assist clients, helping them navigate each stage of the development process, from early target discovery to the preclinical phase of research and development.
What is CAR-T cell therapy?
Chimeric antigen receptor T (CAR-T) cell therapy has rapidly developed in recent years. Unlike conventional cancer therapies, CAR-T therapy is referred to as a "living drug" that uses the patient's immune T cells to attack cancer cells, making it a personalized form of therapy.
The process of CAR-T therapy can be divided into five steps:
- T cells are gathered and isolated from a patient’s peripheral blood (allogeneic donors may also be considered in this stage).
- Using genetic engineering methodologies, T cells are transduced and fitted with a "GPS navigation system" called a CAR, converting T cells into CAR-T cells.
- CAR-T cells are cultured and expanded in vitro to yield sufficient cells for infusion.
- CAR-T cells undergo quality control (QC) testing.
- The prepared high-grade CAR-T cells are injected into the patient to target and kill cancer cells while the normal tissue remains unharmed.
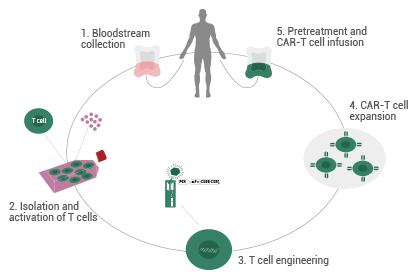
Image Credit: Sino Biological Inc.
Complete solutions for CAR-T cell therapy development
For CAR-T cell therapy, Sino Biological offers an extensive range of solutions for its customers who are engaged in CAR-T cell therapy development. Sino Biological is fully committed to supporting CAR-T research from early CAR development, lentivirus packaging, and CAR-T cell expansion to CAR-T cell quality control processes.
CAR development
CAR is the main component of CAR-T therapy, and acquiring suitable CARs with the capacity to recognize tumor antigens is crucial to CAR development.
Sino Biological offers four antibody development platforms, such as phage display, hybridoma, FACS single B cell, and Beacon® single B cell, to help identify various solutions for scFv discovery.
To expedite the process of CAR-T research, Sino Biological can supply a complete range of high-quality CAR-T target proteins and professional SPR/BLI affinity characterization services for CAR affinity determination and scFv screening.
CAR candidate development
The extracellular domain of CAR encompasses monoclonal antibody-derived single-chain variable fragments (scFv), which plays a role in whether CAR-T can precisely recognize tumor cells. In turn, this plays a key part in the efficacy and safety of CAR-T cells.
Sino Biological offers a diverse range of scFv development platforms suitable for the entire development process, from antigen design and preparation, animal immunization to acquisition of scFv molecules, supporting its pharmaceutical customers and helping them acquire their proprietary scFv sequences.
With a success rate of 90%, Sino Biological’s phage display construction and screening platform delivers high throughput screening of scFv molecules for CAR development.
CAR candidate screening
scFv affinity characterization (BLI/SPR)
scFv is a key element when it comes to the targeted recognition of tumor cells by CAR-T cells, and its affinity and specificity for antigens have a bearing on the efficacy and safety of CAR-T.
Sino Biological offers the SPR/BLI assay platform which can be used to screen scFv molecules efficiently to support CAR-T drug development,.
- Authoritative reports support the IND or BLA filings of CAR-T drugs
- Biacore and ForteBio Octet analysis platforms
- SPR platform is fully accredited by CNAS
Target proteins
scFv screening marks a key phase in CAR development. Sino Biological offers a n extensive range of high-grade CAR-T target protein products to fully support CAR-T research requirements.
- 250+ hot CAR-T target proteins, including fluorescent, site-specific biotinylated, and unconjugated formats
- High activity validated
- High batch-to-batch consistency ensures reproducible results
Assay cell line development
Sino Biological’s assay cell line development services employ lentiviral vectors to create target antigen overexpression cell lines, which can be fine-tuned to carry a variety of markers to meet a customer’s specific needs, thereby supporting the screening of scFv molecules by flow cytometry.
- 15+ years of experience in cell line development
- Complete platform with FACS, qPCR, WB and other identification methods
- Rapid delivery of stable cell lines in 2-4 months
Protein antigen expression services
Target antigens help establish the degree of specificity and effectiveness of CAR. Sino Biological offers one-stop solutions for antigen preparation services ranging from gene synthesis, vector construction, to protein expression, and purification, assisting in the wider development of CAR-T cell therapy.
- Comprehensive QC testing: SDS-PAGE, SEC-HPLC, FACS, ELISA, Cell assay, BLI/SPR, etc.
- Four expression systems: E. coli, mammalian, insect, and CHO/HEK293 stable cell line development
- HTP expression ability, supporting the rapid discovery of CAR-T drugs
CAR-T preparation T cell activation
There is an important step before CAR gen transduction and subsequent CAR-T cell expansion, and that is T cell activation. CD3/CD28 antibodies are the most commonly used for T cell activation in vitro. Sino Biological has developed GMP-grade CD3/CD28 monoclonal antibodies with high purity, high activity, and ultra-low endotoxin (<0.5 EU/mg). These antibodies have been validated at the cell level and can be used to effectively activate and cultivate T cells, supporting the development and production of CAR-T cell therapy drugs.
In CAR-T cell preparation, lentivirus packaging is a vital part of the process followed by the separation and activation of T cells. The titer of lentivirus plays a major role in establishing a positive rate of CAR transfection.
Sino Biological offers a range of important raw materials for lentivirus packaging, such as serum-free HEK293 medium and supplement, as well as SuperNuclease typically used for the removal of nucleic acids during the lentivirus purification process. The company also supplies SuperNuclease ELISA kits for the identification and quantification of residual nuclease impurities.
Lentivirus packaging system
Lentiviral vectors raw materials which are crucial for achieving successful CAR-T therapy. Sino Biological offers transfection reagent, serum-free medium, and supplement for lentiviral production. Sino’s medium has allowed its pharmaceutical customers to complete IND filings with great success.
GMP-grade SMM 293-CD1 medium (cat#: M293CD1)
- Chemically defined, serum-free, and animal component-free
- Suitable for lentiviral packaging to support CAR-T drug development
- Supportive SMS 293-SUPI supplement(Cat#: M293-SUPI)
Nucleic acid removal and detection
Residual host cell DNA presents a safety risk where CAR-T drugs are concerned. Sino Biological’s SuperNuclease helps eliminate residual nuclease contaminants and the supporting SuperNuclease ELISA kit can be used to quantify residual nucleases at trace amounts with great accuracy.
GMP-grade SuperNuclease (cat#: GMP-SSNP01)
- Enhanced specific activity compared to other major manufacturers
- FDA DMF filed (No. 35978), supporting your IND, NDA, and BLA
- High performance: HPLC>99%, enzyme activity >1.1×106 U/mg
- Strict quality control: endotoxin, sterility, mycoplasma, residual HCP, etc.
SuperNuclease ELISA kit (cat#: KIT-SSNP01)
- Broad applicability: detection of residual endonucleases from a range of suppliers
- Full methodological validation reports
- High sensitivity and the lowest detection limit is 14.55 pg/mL
- High batch-to-batch consistency
CAR-T cell expansion
CAR-T cells have the unique ability to recognize specific tumor antigens to exert their anti-tumor activity. Studies have revealed that individual cytokines or cytokine cocktails can boost CAR-T cell proliferation and enhance CAR-T anti-tumor activity. Therefore, the efficacy of CAR-T cells can be significantly improved by choosing the right combination of cytokine cocktails or suitable cytokines for CAR-T cell cultures.
GMP-grade cytokines are often employed as key raw materials during the development process of CAR-T drugs. Therefore, product quality, safety and stability cannot be overlooked. Sino Biological is committed to developing reagents of the highest quality for effective CAR-T cell therapy.
Following strict quality management systems and pharmaceutical-grade release testing standards, Sino Biological implements a stringent pharmaceutical-grade manufacturing environment and production management. This has led to the successful development of a series of high-quality GMP-grade cytokines such as IL-2, IL-7, IL-15 and IL-21 to support the development of cell therapy drugs.
Four systems ensure high quality and GMP-grade cytokines to support CAR-T product manufacturing
- Quality management system
- Quality control system
- Quality assurance management system
- Product safety testing system
CAR-T cell quality control
The quality of CAR-T cells has a direct influence on the clinical treatment effect and patients' health. It may even put patients' lives at risk if not strictly tested. Therefore, CAR-T cells demand strict release testing before being injected or administered into patients. The quality control testing of CAR-T cells typically includes: 1) CAR-T cell purity testing; and 2) CAR-T cell potency and functionality testing.
CAR-T cell purity testing
Source: Sino Biological Inc.
| Test Items |
Purpose |
Solutions |
| Cell Population Profiling |
Monitoring CAR-T cell differentiation, exhaustion, apoptosis, etc. |
A wide range of FACS antibodies, used in:
- CD3+ T cell percentage, CD4+/CD8+ percentage, etc.
- Immunophenotyping: Tn, Tscm, Tcm, Tem
- Activation markers: CD25, CD69
- Exhaustion markers: PD-1
|
| Detection of Target Cells |
Understanding key features of CAR-T products |
- 250+ CAR-T target proteins: high specificity, covering fluorescent, site-specific biotinylated, unconjugated formats
- Protein L: universal detection and low cost
- Anti-idiotype antibody services: specifically designed for scFv, with good specificity
|
Three Solutions for CAR Detection |
|
| Detection of Non-Target Cells |
Detecting percentage of residual tumor cells |
High-quality CAR-T target antibodies, covering CD19, CD20, CD22, EGFR, and CD3 (fluorescence conjugated versions available). |
| Test of Impurities |
Detecting residual cytokines |
IL-2 ELISA Kit (Cat#: KIT11848), IL-7 ELISA Kit (Cat#: KIT11821) |
CAR-T potency and functionality testing
The functional testing of CAR-T cells is a key phase in the quality control (QC) of CAR-T cells, including cytokine release testing, in vitro killing capabilities, and intracellular signal transduction analysis.
Sino Biological has developed high-grade cytokine ELISA kits, which can be utilized for the detection of representative cytokine levels to measure the killing activity and specificity of CAR-T cells on target cells.
Furthermore, Sino Biological has created a series of stable tumor cell lines, which can be used to analyze the killing activity of CAR-T cells in vitro.
ELISA kits
Sino Biological’s high-quality ELISA kits can be used to evaluate the killing activity of CAR-T cells and CRS monitoring after CAR-T cells have been administered to the patients.
- 600+ kits, including human (400+) and mouse (100+)
- High sensitivity and specificity, good stability and reproducibility
- Closely follows eight QC standards to ensure the validity and reliability of the assay results.
Assay cell line development
Sino Biological uses lentiviral vectors to support its over-expression cell line development services which have the capacity to construct Luciferase stable tumor cell lines to assess the killing ability of in vitro of CAR-T cells.
- 15+ years of experience in cell line development
- Complete platform with FACS, qPCR, WB and other identification methods
- Fast delivery of stable cell lines in 2-4 months
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.