Since the mid-1980s, therapeutic monoclonal antibodies have become the fastest-growing class of therapeutics. This was due to the numerous pharmaceutical companies that were trying to find more drug modalities to expand their pipelines.
Moreover, biosimilars currently have more opportunities to enter the market as the patents of a few blockbuster monoclonal antibodies start to expire. In this context, evaluating the safety and efficiency of biotherapeutics has become imperative at the time of the drug-development process.
Specifically, anti-drug antibody (ADA) and pharmacokinetic (PK) assays are performed to study and assess new biosimilars and drug candidates. For PK or ADA assays, anti-idiotype antibodies (Anti-IDs) are powerful tools to quantify the concentration of antibody drugs in patient samples and act as positive controls for anti-drug antibodies (ADA).
The applications of anti-IDs have been emphasized in the article, and the study concludes with the two featured case studies on anti-ID development.
Anti-IDs in pharmacokinetic (PK) assays
Pharmacokinetics (PK) explains and defines the four different phases of a drug in the human or animal body: absorption, distribution, metabolism and excretion (also called ADME). In drug development, PK assays offer necessary data on a drug’s interaction with the body, and also the duration and intensity of its efficacy.
There is a need for comparative PK assays for the development of biosimilar drugs. This helps to calculate possible variations in the biological activity of the reference drug. Depending on various binding modes and properties (Figure 1), anti-idiotype antibodies could be categorized into three types: non-blocking, antigen-blocking and complex specific.
Based on such features, different formats of PK assays can be fixed to quantify free, total or bound antibody drug concentrations in serum.
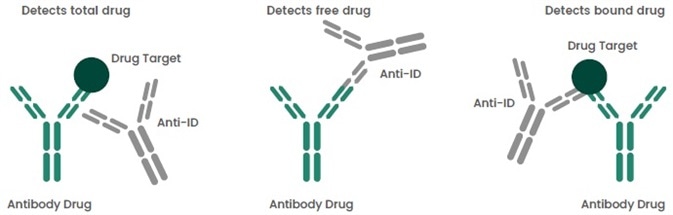 Figure 1. Types of Anti-IDs in PK Assays. Image Credit: Sino Biological Inc.
Figure 1. Types of Anti-IDs in PK Assays. Image Credit: Sino Biological Inc.
Anti-IDs could be utilized to detect and measure antibody drug levels in animal or human serum and are considered crucial detection reagents for PK studies. There is a range of analytical techniques for quantitative analysis of antibody drugs, with ELISA being the most generally utilized format.
In an anti-ID capture ELISA (Figure 2), anti-IDs are coated to a plate and further samples comprising the antibody drug are added to the system. Furthermore, the antibody drug is measured with labeled anti-IDs that bind to the idiotype of the drug.
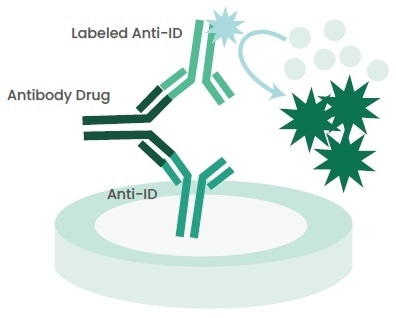
Figure 2. Principle of Anti-ID Capture ELISA. Image Credit: Sino Biological Inc.
Anti-IDs in immunogenicity/anti-drug antibody (ADA) assays
Anti-drug antibodies (ADA) detection is an important part of the immunogenicity assessment process for therapeutic proteins like monoclonal antibodies, ADCs and fusion proteins. A multi-tiered testing approach (Figure 3) is commonly used in these situations.
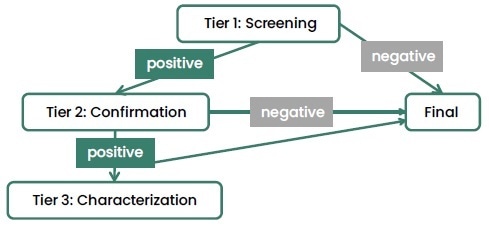
Figure 3. Multi-Tiered Testing Approach. Image Credit: Sino Biological Inc.
The method makes use of a sensitive screening assay to determine positive antibody samples, a confirmatory assay to reduce false-positive results and finally a characterization assay to evaluate the neutralizing capacity of antibodies.
Throughout the entire process, anti-idiotypic antibodies are considered vital reagents.
Various assays could be utilized to detect ADAs, such as radioimmunoprecipitation assay (RIPA), ELISA, surface plasmon resonance (SPR), and electrochemiluminescence (ECL). Amongst these, bridging ELISA is the most generally utilized technique enabling the detection of all isotypes of ADAs (IgM, IgG, IgA, etc.).
In a normal bridging ELISA, the antibody drug has been pre-coated on a plate, and the labeled antibody drug is incubated with patient samples to test the existence of ADAs (Figure 4). Anti-IDs will be utilized as positive controls or reference standards for qualitative analysis of ADAs in samples.
 Figure 4. Principle of ADA Bridging ELISA. Image Credit: Sino Biological Inc.
Figure 4. Principle of ADA Bridging ELISA. Image Credit: Sino Biological Inc.
Anti-idiotype antibody generation packages
To expedite the drug development of users, Sino Biological offers a complete range of custom anti-idiotypic antibody production services, from antigen preparation, anti-idiotype antibody development to detection method establishment as well as kit development.
Moreover, the team has extensive expertise and experience in targeting a wide range of modalities, including full-length mAb, Fab, F(ab')2, scFv, VHH, ADCs, bsAbs and Fc-fusion proteins. Tailored anti-idiotypic antibodies could be utilized to fix pharmacokinetic (PK)/ADA assays to identify particular antibody drugs or ADA levels in the samples.
Table 1. List of Anti-Idiotype Antibody Services. Source: Sino Biological Inc.
| Service |
Deliverables |
Timeline |
| Anti-ID Rabbit pAb Service |
• Purified antibodies
• CoA |
2-3 months |
| Anti-ID Mouse mAb Service |
• Positive clones, 2 tubes cells/clone
• Purified antibodies, 10 mg/clone
• ELISA antibody pair (optional)
• CoA |
4-6 months |
Case study #1: The development of anti-IDs targeting BsAbs
Bispecific antibodies (BsAbs) are genetically engineered antibodies consisting of two specific antigen-binding sites that tend to identify two different antigens or epitopes. When compared with monospecific antibody drugs, BsAb drugs frequently have an increased tendency for aggregation, proteolysis and physical instability, etc.
Moreover, the binding of two different antigen-binding sites to their equivalent ligands might have large steric hindrance effects on each other. Keeping these factors in mind, it is very hard to screen anti-IDs against various antigen-binding epitopes of bispecific antibodies.
Sino Biological has well-understood those barriers and has also been successful in completing various anti-ID development projects that recognize BsAb drugs.
These produced anti-IDs have the ability to bind to two different antigen-binding sites respectively, with great specificity. As displayed in Figure 5, seven anti-idiotype mouse monoclonal antibody clones displayed highly specific binding to the BsAb drug and showed no binding to total human IgG or human IgG isotype control.
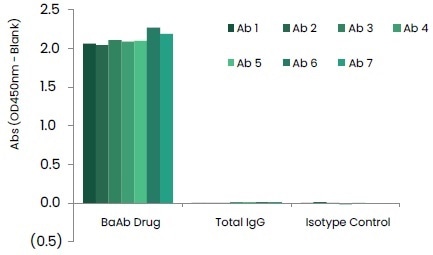
Figure 5. Binding Specificities of Anti-ID mAbs. Image Credit: Sino Biological Inc.
Additionally, screening a panel of neutralizing and non-neutralizing anti-IDs against the monospecific unit A and B of BsAb drugs was also done.
Furthermore, antibody pairing was executed in capture ELISA format. In various concentrations of human serum, the detection sensitivity was obtained at the pg level, and the recovery rate ranged from 80 to 120%. Figures 6 and 7 show the data associated with these.
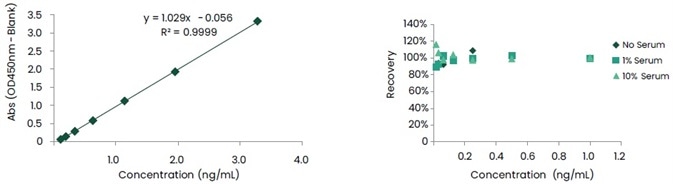 Figure 6. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit A. Image Credit: Sino Biological Inc.
Figure 6. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit A. Image Credit: Sino Biological Inc.
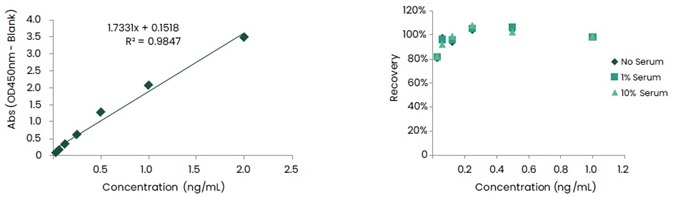 Figure 7. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit B. Image Credit: Sino Biological Inc.
Figure 7. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit B. Image Credit: Sino Biological Inc.
Case study #2: Generation of anti-IDs targeting scFv/VHH
scFv is known as a fusion protein of the immunoglobulin heavy and light chain variable regions, which are linked by a peptide linker. VHH is a single variable domain situated on a heavy chain, also called a nanobody. The features of scFv and VHH include high specificity, small size, high stability and strong tissue penetration.
However, due to their low immunogenicity, the development of anti-scFv/VHH antibodies remains a hard one.
For this technical challenge to be fulfilled, Sino Biological launched the scFv/VHH immunization platform. The success rates of immunizing mice with VHH and scFv reached 80% and 100%, respectively.
Furthermore, a quick immunization protocol was launched for VHH, making it possible for mice immunization to be done in 37 days. Figure 8 reveals that the titers of VHH mice are sufficient to use for subsequent cell fusion. Rapid immunization has displayed comparable titer levels in contrast with regular immunization.
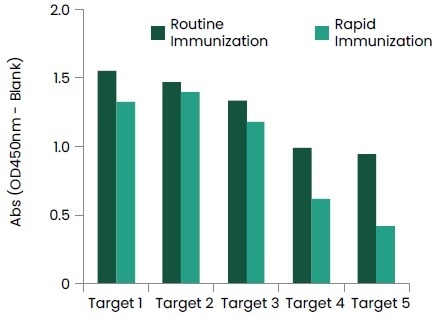
Figure 8. Titer Check of VHH Immunized Mice. Image Credit: Sino Biological Inc.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.