Reagent purity has a direct impact on the reliability of experimental results in biomedical research and biomanufacturing, as well as the safety of downstream therapeutic products.
Endotoxins are a common pollutant in protein preparations. Even minute levels of endotoxin in recombinant proteins can cause strong immunological reactions, distorting results and jeopardizing patient safety. This is particularly true in sensitive applications like immunology, cell and gene therapy, and vaccine manufacture.
Sino Biological has launched ProPure™, an advanced line of endotoxin-free recombinant proteins, to meet this urgent need.
What are ProPure™ endotoxin-free proteins?
ProPure™ is a new industry standard for producing recombinant proteins with reduced endotoxin concentration. ProPure™ products are designed to achieve endotoxin levels below the limit of quantification (LOQ), setting them apart from traditional methods.
Produced in the United States, at Sino Biological's cutting-edge Center for Bioprocessing (C4B) in Houston, Texas, this facility uses mammalian expression methods and patented multi-step purification protocols. Using these techniques, they produce proteins of high purity, stability, and biological integrity.
ProPure™ endotoxin-free guarantee: Sino Biological's assurance of quality
Ensuring minimal endotoxin levels at every step of protein production
- Endotoxin-free plasmids
- Endotoxin-free buffer with filtration
- Regular clean-in-place (CIP)
- Low endotoxin affinity plastic containers
Importance of endotoxin-free proteins
Endotoxin contamination can jeopardize sensitive studies and medicinal uses.
- Even small quantities of endotoxin can activate immune pathways, causing false signals in cell-based assays and immunological research.
- Contaminated proteins used in animal studies or clinical research might cause adverse inflammatory reactions, posing a risk to animal welfare and patient safety.
- Endotoxin control is essential for biomanufacturing and therapeutic protein production to comply with regulations and assure product safety.
Features and advantages of ProPure™
- Ultra-Low Endotoxin Content: Advanced purification processes reduce endotoxin levels to well below the limit of quantification (LOQ), exceeding industry standards and reducing experimental risk.
- Mammalian Expression Systems: These systems and customized techniques lower bacterial endotoxin input, resulting in proteins with native-like PTMs, folding, and functioning.
- High Consistency and Quality: Sino Biological’s patented manufacturing process provides consistent and reproducible outcomes in sensitive areas, including immunology, vaccine research, and animal investigations.
- Customization Options: ProPure™ offers customized proteins to meet specific research and manufacturing needs, in addition to regular catalog proteins.
- Made in the USA: Sino Biological's C4B facility in the United States demonstrates the company's dedication to provide the global life sciences community with fast production, dependable logistics, and quality assurance.
Case study
The Human B7-H3/CD276 protein is an excellent example of endotoxin-free protein quality, since it achieves above 90 % purity using SDS-PAGE and SEC-HPLC. Its purity and accurate molecular size (about 60.4 kDa by SEC-MALS) are confirmed and its bioactivity is confirmed by ELISA (EC50 = 6.7 ng/mL).
This case emphasizes the significance of comprehensive testing for purity, identity, and bioactivity in ensuring high-quality, contamination-free proteins for sensitive research.
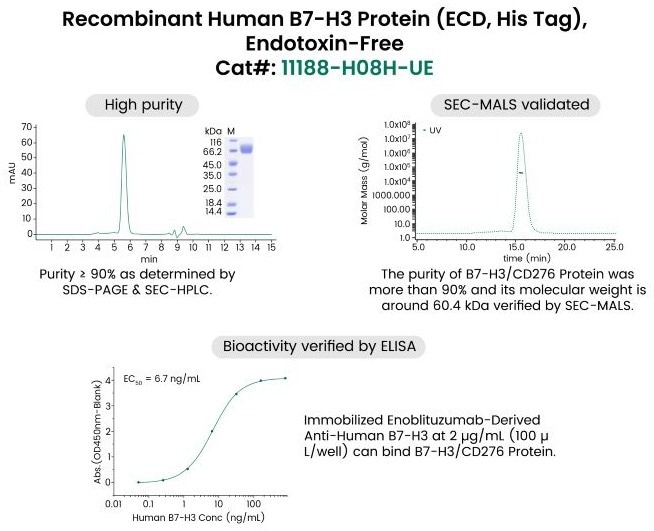
Fig. 1. Premium ProPure™ Endotoxin-Free Protein. Image Credit: Sino Biological Inc.
Customer-centric innovation
Sino Biological's strategy extends beyond supplying ultrapure proteins. The C4B also collaborates with research laboratories and biotech companies to provide:
- Custom protein production: Customized to match research needs, including production volume, tag design, and formulation.
- Fast turnaround: Sino Biological’s cutting-edge technology, expertise in recombinant production, and fast shipping ensure shorter lead times and increased reliability for critical projects, such as pandemic response and immunotherapy development.
- Technical support: Sino Biological’s expert assistance and support help customers maximize experimental success.
Why choose C4B for endotoxin-free protein production?
- Optimized mammalian expression platforms and rigorous QC results in high quality and consistency.
- Customized proteins and antibodies delivered in as little as two weeks.
- 24-well plates to 25 L bioreactors for screening to production protein requirements.
- State-of-the-art equipment and minimized endotoxin levels throughout processing.
Impact and industry recognition
Sino Biological's ProPure™ sets a new standard in the life sciences industry.
- Researchers and biomanufacturers now have a reliable, high-quality source of recombinant proteins, which improves accuracy, reproducibility, and safety and leads to scientific breakthroughs.
- The release of the ProPure™ product line signifies Sino Biological’s ongoing investment in both technological innovation and service to the global scientific community.
Conclusion
In today's world of advanced biomedical research and therapies, using fully endotoxin-free proteins has never been more crucial.
Sino Biological's ProPure™ endotoxin-free recombinant proteins help researchers and industry professionals achieve scientific excellence while avoiding risk.
As research becomes ever more sophisticated and regulatory requirements tighten, ProPure™ is a game-changer for life science applications, ensuring safety, consistency, and scientific integrity.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies, and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce up to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.