Sponsored Content by Emulate, Inc.Reviewed by Olivia FrostAug 5 2025
In this interview, NewsMedical speaks with Jim Corbett, CEO of Emulate, Inc., about the development and impact of the AVA Emulation System and how it helps scale organ‑chip technologies to improve decision-making in preclinical drug discovery and support regulatory acceptance.
To begin, what was the original problem or unmet need in the field that led to the creation of AVA, and how did the concept take shape from there?
With nearly 90 % of candidate drugs that enter clinical trials failing to receive FDA approval, there is an urgent need to improve the drug discovery and development process. Organ-on-a-Chip technology has emerged as a promising alternative, but a lack of scalability has hampered adoption. AVA is the first self-contained Organ-on-a-Chip workstation to fuse high-throughput culture, environmental control, and real-time imaging into a single unit. Supporting up to 96 Organ-Chip Emulations in a single run, AVA enables faster, more confident decision-making and lowers the cost per replicate.

Image Credit: Adisak Riwkratok/Shutterstock.com
AVA is designed to support human-relevant research at scale. How does the platform enhance the validation of Organ-on-a-Chip data, and why is that validation essential for building regulatory trust?
The ultimate goal is to obtain more human-relevant data. For that data to be trusted, it must be accurate and reproducible, which requires large-scale studies. AVA enables up to 96 samples per run so that users can generate predictive values for risk assessment. Confidence will expand as more human cell donors and modalities are tested.
Your 2022 study on the Emulate Liver-Chip, which the FDA referenced in its recent roadmap, showed superior predictive power over animal models. How did that study influence AVA's development and credibility in regulatory discussions?
The 2022 study showed that the Liver Chip correctly identified seven out of eight hepatotoxic drugs and had a 100 % success rate in distinguishing structural analogs. It became the first Organ Chip accepted into the FDA's ISTAND program. AVA includes equivalency studies to show comparable detection of drug-induced liver injury, and its higher throughput will help build confidence in regulatory communities.
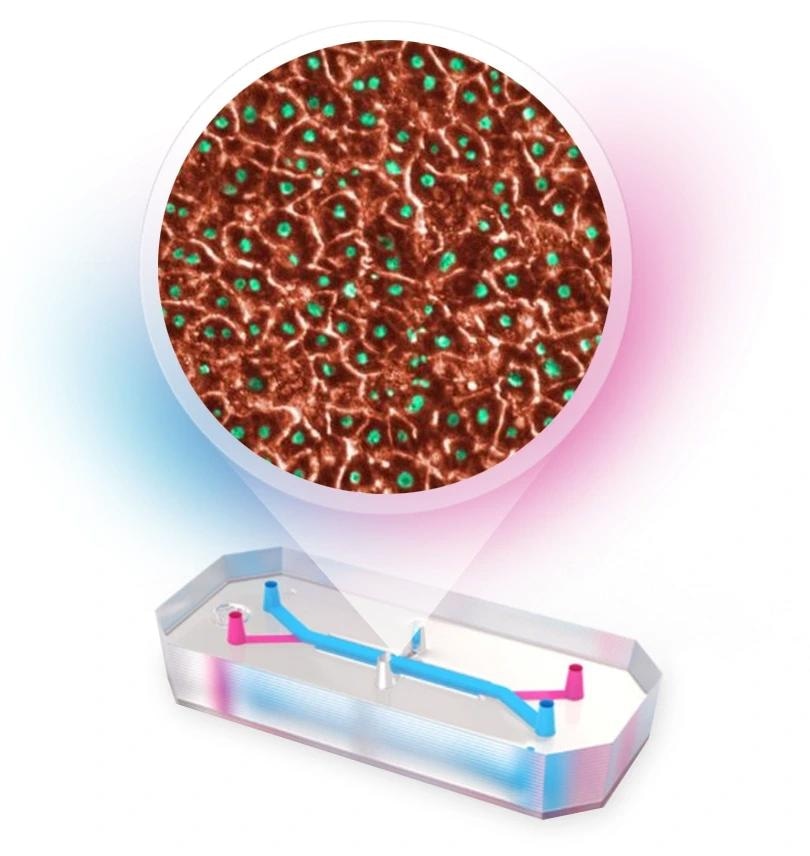
Emulate human Liver-Chip
Image Credit: Emulate
The FDA now offers expedited review for IND submissions, including new approach methodologies like Organ-Chips. How can AVA help pharmaceutical companies capitalize on these new regulatory incentives?
AVA meets the requirements of biological fidelity and throughput, empowering teams to rank-order candidates, pinpoint off-target toxicities, and advance safer therapies. It also enables synergy with other NAMs by combining in vitro, in silico, and omics data to support safety decisions and align with regulatory goals.
With the NIH's recent move to stop funding animal-only studies, academic researchers are under new pressure to adopt human-based models. How does AVA support this transition and help academic labs scale their use of Organ-on-a-Chip technologies?
Academia has already embraced Organ-on-a-Chip technology, but the cost of consumables was a limiting factor. AVA reduces the cost per sample by over 75 %, making experiments more economically feasible. It's also an ideal fit for core labs running smaller experiments together.
High-throughput analysis and reproducibility have been persistent challenges in this space. What specific features of AVA help address these issues and make Organ-Chip data more accessible and scalable?
AVA integrates and automates imaging workflows, generating data while the chips are under flow with consistent conditions. This increases intra- and inter-experiment reproducibility and streamlines analysis.
Looking ahead, how do you see AVA shaping the drug development process, especially in improving how efficacy and toxicity are assessed in early-stage research?
With AVA's introduction and growing Organ-Chip maturity, we expect deeper integration into early-stage research. Moderna used the Liver Chip to screen lipid nanoparticles before animal testing, reducing cost and accelerating results. Organ chips may soon become the starting point for human-relevant models and influence regulatory decisions across the pipeline.

Image Credit: Emulate
As human-relevant methods gain regulatory traction, how important is standardization across platforms like AVA? Are efforts underway to align data formats and validation practices across the field?
Standardization is important. Groups like NIST, Critical Path Institute, 3Rs Collaborative, Joint Research Center, and CEN/CENELEC are working to align data formats. Platform standardization will likely come later, as early requirements can hinder innovation.
Despite recent progress, what barriers prevent the broader adoption of non-animal methods, and how can technologies like AVA help remove those barriers?
Two hurdles remain: regulators need large, reproducible data sets, and those data must fit existing pipelines. AVA automates multi-chip experiments, runs industry-standard protocols, and streams harmonized readouts into analytics platforms. This enables companies to meet regulatory expectations without reinventing workflows.
What do you find most promising about the current convergence of regulatory change and technological innovation, and how close are we to seeing Organ-on-a-Chip systems become the default tool in drug testing and development?
The FDA and NIH announcements shift from permission to expectation for human-relevant data. Organ-Chips uniquely recreate the full-organ context of human physiology. With regulators opening the door and innovations like AVA automating workflows, these systems will accelerate ethical, sustainable drug discovery.
Discover AVA today
About Jim Corbett
Jim Corbett is the CEO of Emulate, Inc., a leader in Organ-on-a-Chip technologies. He has held senior executive roles across global biotechnology and life sciences companies, including PerkinElmer, where he served as Executive Vice President and President of Discovery & Analytical Solutions. His background spans analytical instruments, diagnostics, and medical imaging. Corbett brings deep commercial and operational expertise from Fortune 100 companies and startups, and he has guided Emulate in scaling Organ-Chip technologies for pharmaceutical and regulatory use. His leadership reflects a strong commitment to replacing animal testing with more accurate, human-relevant biomedical research and drug development models.
About Emulate, Inc.
At Emulate, we understand that animal studies and reductionist models are limited because they are not based on integrated human biology.
By leveraging 21st century technologies, we are able to overcome these limitations with living human in vitro models that empower researchers to explore the biological mechanisms of health and disease. These microphysiological systems (MPS), commonly known as Organ-Chips, are setting a new standard for how we study biology and develop drugs, therapies, and cures for those who need them most.