Key Points
- The German Fritz Stephan GmbH is one of the top global developers and producers of respiratory apparatus and anesthetic systems
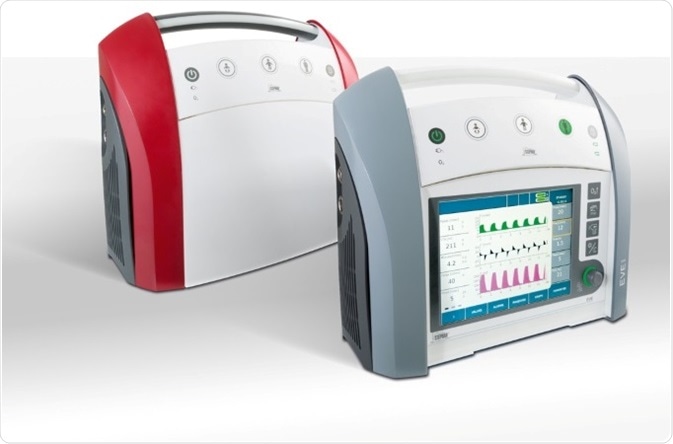
- The company’s EVE mobile respiratory instrument has reached new heights in technology
- EVE is certified for the whole chain, from emergency transport (position of incident) to intensive care
- It is no longer necessary for patients to be linked to different respiratory devices while being delivered to the hospital and operating room
The Challenge
The EVE mobile respiratory device needed a compact sensor solution that is insensitive to ambient conditions and can accurately measure even tiny rates of flow.
The Solution
Typically, the specifications of the application would have necessitated a standard mass flow sensor, but instead, First Sensor unveiled the newly designed differential pressure sensors of the LDE/LME series to the team of developers.
The LDE/LME series uses MEMS sensor technology. The sensors offer a minute flow channel built into the semiconductor chip and measuring just 2 x 2 mm. In this micro flow channel, a heating element is placed amid two resistors which are sensitive to temperature. Even a minuscule gas flow alters the temperature profile and generates a voltage signal relative to the mass flow.
The Success and the Benefits
The LDE/LME differential pressure sensors are tiny, extremely precise and are not responsive to ambient conditions. These are clear advantages, particularly with this mobile application. Thanks to the extreme sensitivity of the LDE/LME sensors, EVE has such precise levels of measurement that it can dependably distinguish even the frequently tiny respiratory flows of newborn and premature babies.
The counterbalance of the LDE/LME sensors is set via measurement technology, meaning that it is not necessary to zero the signal for calibration every four seconds, as is required with standard sensors. This not only guarantees accurate long-term constancy, but moreover, could lower production expenses by excluding the components needed for calibration and zeroing, including four valves.
First Sensor is compliant with the exacting standards required for medical goods, as per ISO 13485, allowing customers like Fritz Stephan to have their own developments certified.
How was the Cooperation?
From a supply chain point of view, we depend on partners who tune in to our market. Most medical technology products are sold in small to moderate quantities, but for a very long period. Even small changes in design and components result in elaborate approval processes. First Sensor also supplies smaller quantities in line with requirements, and provides long-term availability for the installed sensors.
As soon as our developers have specified the product idea and the requirements for the sensor system, we contact our development partner First Sensor. Then we discuss as a team how we can meet these requirements and which sensors we should use. Of course it helps if the partner company and their experts know our processes and requirements, have professional experience and really use their own mind. This way we quickly find out if a standard sensor is adequate, or if adjustments or even a new development are required
Jens Amberg, Project Manager Development, Fritz Stephan GmbH, Germany.

First Sensor AG
First Sensor AG is one of the world's leading suppliers in the field of sensor systems. Our company develops and manufactures both standardized and tailor-made sensor solutions for the detection of light, radiation, pressure, flow, level and acceleration for applications in the Industrial, Medical and Mobility growth markets.
The company produces in-house and along the value-added chain from component to system level.
With over 800 employees, we are represented at six German locations and also have development, production and sales sites in the USA, Canada, China, the Netherlands, Great Britain, France, Sweden and Denmark along with a worldwide partner network. We guarantee our compliance through regular successful certifications of the sites according to ISO/TS 16949, ISO 14001, EN ISO 13485, EN 9100 and ISO 9001 - matching the respective business field.
First Sensor AG is listed in the Prime Standard of the German stock exchange in Frankfurt.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.