Pharmaceutical manufacturing and quality control processes are stuck in the past. Process Analytical Technology (PAT) can bring production systems up-to-date and help realize manufacturing efficiency and cost savings. This article outlines the application of Raman spectroscopy as a PAT for crystallization monitoring and control in the pharmaceutical industry.

Image credit: pixabay.com/HeungSoon
Quality control is vital throughout drug production processes. Well-designed, reliable, and robust analytical strategies are crucial for producing safe medicines in a cost-effective manner.
Despite technologies available that could provide constant monitoring, most quality assurance testing was performed on grab samples collected during the synthesis process because it was the only analysis with regulatory guidance. In the past, stringent regulatory requirements and long discovery and development times limited the use of tools that could provide real-time process information and most drug manufacturing processes seem to be frozen in time.
Process Analytical Technology Provides a Framework for Innovation in Pharmaceutical Manufacturing
To address industry-wide issues with manufacturing quality and efficiency, the FDA issued guidelines in 2004 to encourage innovation in pharmaceutical development, production, and quality assurance.1 The regulatory framework, known as Process Analytical Technology (PAT), encourages the development of continuous, automated processes, with on-line or in-line quality assurance and constant feedback. Since its implementation, the PAT framework has helped the pharmaceutical industry to reduce production cycling times, prevent rejection of batches, improve energy efficiency, and reduce material waste.
Crystallization Control is a Vital Part of Drug Production
Solid-state pharmaceuticals are generally preferred as they are more stable and easier for patients to take. However, pharmaceuticals and biopharmaceuticals active ingredients are often produced in solution phase. The final solid-state of a drug is then formed using a crystallization process, a common procedure in the pharmaceutical industry.2,3
Crystallization is complex and can be influenced by a wide range of factors, including temperature, pH, and the presence of impurities. As the crystalline form of a drug affects its functionality, solubility, purity, and stability, crystallization must be precisely controlled.2,3
Process Analytical Technology for Crystallization
Applying PAT to the crystallization of drugs can ensure product quality and allow real-time process adjustments. Spectroscopy-based PAT techniques are ideal for in-line monitoring as they use light to provide information about the state of the drug. The use of light to analyze the drug provides fast, non-destructive, and non-invasive chemical analysis. Fiber-optic cables coupled to reaction vessels can provide remote spectroscopic measurements and continuous monitoring.3
Raman Spectroscopy is Ideal for Monitoring Crystallization
Raman spectroscopy involves illuminating a sample with monochromatic light. The light interacts with the sample, and the resulting shift in energy gives information about the vibrational modes in the system. Raman spectroscopy provides a molecular fingerprint, enabling identification of the substances in a sample.
Raman spectroscopy has several advantages over other spectroscopic techniques. It is compatible with solids, liquids, gases, and turbid media, and provides high chemical specificity and good reproducibility. Raman spectroscopy can also provide a molecular understanding of the crystallization process, confirm that crystallization is complete, and characterize the final solid form of the drug.4,5
A limitation of Raman spectroscopy is the inherent weakness of the Raman scattering process, resulting in low sensitivity. However, the high concentrations needed for crystallization means that low sensitivity is not usually a problem.4,5
Continuous feedback loops using Raman spectroscopy have already been applied to industrial upstream biopharmaceutical processes, including cell culture and fermentation. Feedback control loops using in-line technologies like Raman spectroscopy provide efficiency savings in less than a year of implementation.4,5
Raman Spectroscopy in Small Molecule Crystallization
Crystallization monitoring and process control using Raman spectroscopy has already been demonstrated in the industrial manufacture of several small molecule pharmaceutical products.6-11
One example, progesterone, has two polymorphs. During synthesis, progesterone crystallizes as the kinetically favored polymorph first (Form II) and then recrystallizes as the thermodynamically more stable polymorph (Form I). Monitoring the process with Raman spectroscopy enables crystallization conditions to be optimized to produce the preferred polymorph.6
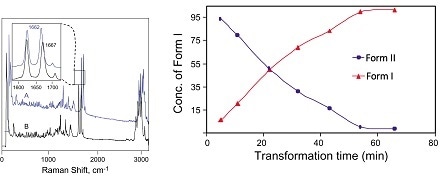
Figure: Raman spectra of Form I and Form II of solid-state progesterone (left). Concentrations of Form I and Form II polymorphs during crystallization monitored using Raman spectroscopy (right). Image credit: Kaiser Optical Systems, Inc.6
Raman Spectroscopy in the Crystallization of Biopharmaceutical Products
Raman spectroscopy has a rich history in the analysis of biological molecules and has been widely used in protein analysis and biomedical research. As a result, Raman spectroscopy is an excellent option for monitoring biopharmaceutical crystallization.4,5
For biopharmaceuticals, other limitations include overlapping Raman spectra from media components and complex biological structures, and the presence of fluorophores in biopharmaceuticals, which can cause interference. However, advanced instrumentation and robust experimental design makes it possible to exploit the advantages of Raman spectroscopy in crystallization monitoring in an industrial context.4,5
Researchers from the University of Puerto Rico used in-line Raman spectroscopy to monitor the crystallization of a lysozyme model protein. The effects of temperature, time, and NaCl concentration on the crystallization process were monitored and the researchers used the results to determine to optimal crystallization conditions.6,12
Kaiser Optical Systems Inc. is a World Leader in Raman Spectroscopy
Application of in-line Raman spectroscopy techniques to manufacturing environments can be achieved using currently available Kaiser Raman instrumentation. Transferring the analytical model from the laboratory to large-scale processes can be challenging, but common analysis technology and probe hardware can significantly ease the transfer process. Kaiser supplies a range of robust, reliable, and high-performance Raman spectroscopy solutions including instrumentation that can be used in laboratory research and industrial manufacturing.13
Kaiser also offers a versatile range of Raman sampling options, including probes for in-line measurements in both the laboratory and in large-scale manufacturing. Their fiber optic probes allow your Raman solution to be added to your existing configuration, so customizing or re-purchasing equipment is not required to accommodate in-line Raman analysis.14
References and Further Reading
- ‘Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance’ https://www.fda.gov/media/71012/download
- ‘Pharmaceutical Crystallization’ — J. Chen, B Sarma, J.M.B Evans, A.S. Myerson, Crystal Growth & Design, 2011, 11(4):887-895.
- ‘Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry’ — Z. Gao, S. Rohani, J. Gong, J. Wang, Engineering, 2017, 3(3):343-353.
- ‘Applications of Raman Spectroscopy in Biopharmaceutical Manufacturing: A Short Review’ — Buckley K, Ryder AG, Applied Spectroscopy, 2017, 71(6): 1085-1116.
- ‘Raman Spectroscopy as a Process Analytical Technology for Pharmaceutical Manufacturing and Bioprocessing’ — K.A. Esmonde-White, M. Cuellar, C. Uerpmann, B. Lenain, I.R. Lewis, Analytical and Bioanalytical Chemistry, 2017, 409(3):637-649.
- ‘Raman Spectroscopy in Crystallization’ — K.A. Esmonde-White, Kaiser Optical Systems Inc, 2019.
- ‘A Calibration-Free Application of Raman Spectroscopy to the Monitoring of Mannitol Crystallization and Its Polymorphic Transformation’ — H. Hao, W.Su, M. Barrett, V. Caron, A. Healy, B. Glennon, Organic Process Research & Development, 2010, 14(5):1209-1214.
- ‘Analysis of the Crystallization Process of a Biopharmaceutical Compound in the Presence of Impurities Using Process Analytical Technology (PAT) Tools.’ — E. Simone, W. Zhang, Z.K. Nagy, Journal of Chemical Technology & Biotechnology, 2016, 91(5):1461-1470.
- ‘Evaluation of Solid Dispersions on a Molecular Level by the Raman Mapping Technique’ — N. Furuyama, S. Hasegawa, T. Hamaura, S. Yada, H. Nakagami, E. Yonemochi, K. Terada, International Journal of Pharmaceutics, 2008, 361(2-1):12-18.
- ‘Physical Stability and Recrystallization Kinetics of Amorphous Ibipinabant Drug Product by Fourier Transform Raman Spectroscopy’ — W. Sinclair, M. Leane, G. Clarke, A. Dennis, M. Tobyn, P. Timmins, Journal of Pharmaceutical Sciences, 2011, 100(11):4687-4699.
- ‘In-Line Monitoring of Carvedilol Crystallization Using Raman Spectroscopy’ — H Pataki, I. Markovits, B. Vajna, Z.K. Nagy, G. Marosi, Crystal Growth & Design, 2012, 12(11):5621-5628.
- ‘Design and In-Line Raman Spectroscopic Monitoring of a Protein Batch Crystallization Process.’ — J. Mercado, M. Alcalà, K.M. Karry, J.L. Ríos-Steiner, R.J. Romañach, Journal of Pharmaceutical Innovation, 2008, 3(4):271-279.
- ‘Raman Spectroscopy’ https://www.uk.endress.com/en/field-instruments-overview/measurement-technologies/raman-technology
- ‘Raman Probes/Sampling’ https://www.endress.com/en/field-instruments-overview/optical-analysis-product-overview/raman-rxn-20?t.tabId=product-overview
About Kaiser Optical Systems, Inc.
Kaiser Optical Systems, Inc. is a world leader in spectrographic instrumentation and applied holographic technology. Principal products include Raman sensors and instrumentation, advanced holographic components for spectroscopy, and astronomy and ultra-fast sciences. Principal offices and the manufacturing facility are located in Ann Arbor, Michigan.
Our products and services are deployed throughout the world in such diverse applications as pharmaceutical and chemical manufacturing, nanotechnology, telecommunications, education, forensic science, deep-sea exploration, and astronomy. From particles smaller than a human hair to objects as large as planets, our products are providing our customers unique insights into both today’s as well as “age-old” questions.
Kaiser was founded in 1979 to meet the need for diffractive or holographic optics for the avionics market. Kaiser entered the spectroscopy market in 1990 with the introduction of the holographic notch filter. In 1993 Kaiser released their first Raman analyzer, the HoloProbe. In 2013, the company became part of the Endress+Hauser Group.
To better serve the European community, Kaiser opened a new subsidiary in Europe in 1998. Kaiser Optical Systems SARL is located in Lyon, France. Kaiser SARL supervises our distributor network within Europe.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.