Key Issues
- Taste, texture and color are three quality attributes of salmon fish meat
- Raman spectroscopy gives information on all three fish quality attributes quickly and non-destructively, and can be performed in the laboratory or in the processing plant
- Laboratory measurements of fish quality involve destructive sample preparation, time-intensive wet chemical or chromatographic analysis, and are not compatible with processing plant speeds
Introduction
There is high consumer demand globally for salmon, as it is a popular fish. This demand has generated a requirement for efficiency from the raising of salmon to its processing in the plant. Rapid measurements of fat, firmness and color, the three main elements of fish quality, can aid in reaching the goal of efficient processing. Fat content is related to taste and mouth feel.1
Salmon meat color effects consumers’ view of fish quality, and consumers favor a deep pink color.2 Firmness impacts consumer approval of both smoked and raw salmon products.3 These three quality features have been conventionally measured in the laboratory using titration and chromatographic methods or, when measuring color, by visually inspecting against a color card. Titration and chromatographic techniques are the “gold standard” in measuring these features. However, they are not open for use in inline measurements, need destructive sample preparation and take hours to finish measurements.
The growing demand for salmon is powering the use of new analysis technologies that can keep up with automated processing plants to offer a quick and accurate assessment of fish quality in real-time and inline.
Vibrational spectroscopies like near-infrared (NIR) spectroscopy and Raman spectroscopy work well in achieving non-destructive, quick, data acquisition. While NIR has been demonstrated to be a dependable technique for measuring moisture and fat content in salmon, those features only offer information on texture. Raman spectroscopy provides the possibility of analyzing firmness, fat and color features simultaneously, as well as perhaps examining the interactions between these attributes.
Methods and materials
Raman spectra were gathered from store-bought samples of Atlantic salmon, smoked salmon and wild coho. Two experiments were conducted. In the initial experiment, laboratory tests were conducted utilizing a Kaiser Raman analyzer operating at 1000 nm equipped with a small-area (measured area ~100 μm2 ) contact probe. The probe was placed by hand on the fish’s surface and signal was collected for 10-60 s. In the second experiment, a Kaiser Raman analyzer operating at 785 nm fitted with a Ph AT large volumetric non-contact probe (measured volume ~3-6 mm3 ) was utilized to evaluate compatibility with processing plant conditions. Signal was collected for 1-5 s. Spectra are exhibited without any preprocessing.
Results and discussion
The first tests conducted on store-bought salmon suggested feasibility of 1000 nm excitation for laboratory measurements. With the small-area probe it was possible to collect signal from each of these zones without substantial interference from neighboring zones. Figure 1 displays the representative Raman spectra from Atlantic salmon, where muscle-rich and fat-rich zones can be clearly seen.
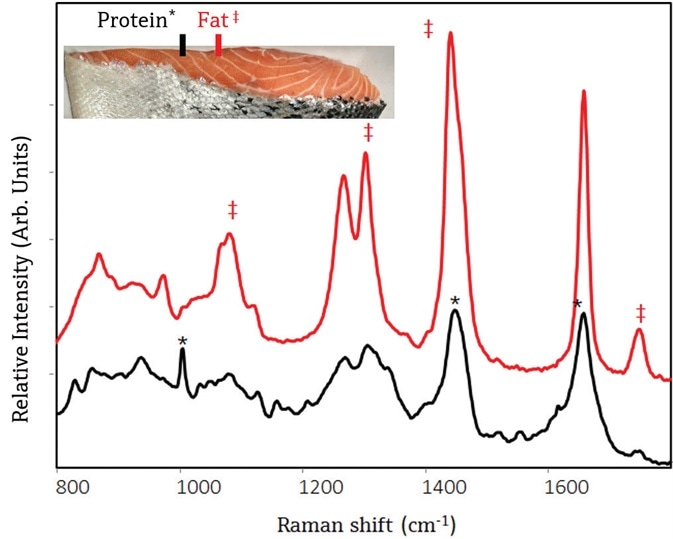
Figure 1. Raman spectra of Atlantic salmon using a small-area probe shows different chemical composition of meat and fat zones within the salmon. The top spectrum was collected from a fat zone, and spectral attributes unique to lipids are shown with a ‡. The bottom spectrum was collected from a muscle zone, and spectral attributes unique to muscle proteins are shown with a *. Image Credit: Kaiser Optical Systems, Inc.
The Raman spectra are also visibly different. The top spectrum contains attributes from mostly lipids in fat, and the bottom spectrum contains protein attributes. The approach of using a small-area contact probe would be valid when more accurate zonal measurements are needed for quality control purposes. Significantly, there were no bands seen from carotenoid pigments in spectra from Atlantic salmon. Contrarily, spectra of wild coho salmon contained signal from carotenoid pigments, as well as lipid and protein bands (data not displayed).
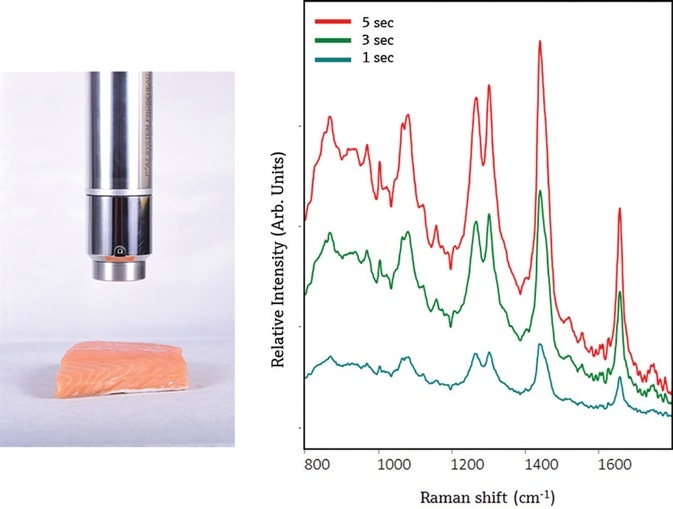
Figure 2. The PhAT probe provides a non-contact measurement of Atlantic salmon (left). The resulting Raman spectra show primarily contributions from lipids, as evidenced by the presence of narrow lipid bands at ~ 1750 cm-1 and 1301 cm-1, a narrower Amide I envelope at ~1650 cm-1, and a shifted CH3 envelope to ~1441 cm-1. Spectra were not preprocessed and are offset to enable clear visualization of each spectrum. Image Credit: Kaiser Optical Systems, Inc.
Mock process measurements were conducted on Atlantic salmon. The PhAT probe offers a non-contact measurement above a large volume, and the resulting spectra had contributions from the muscle and fatty tissue portions. To be compatible with conveyor belt speeds, measurement times were adjusted. As illustrated in Figure 2, Raman spectra collected at 1s, 3s, and 5s are suitable for input into a multivariate or univariate model with decent signal-to-noise and spectral resolution. No carotenoid bands were seen in spectra from Atlantic salmon, like measurements gathered with a contact probe.
Conclusions
These results demonstrate the utility of Kaiser Raman spectroscopy to offer a multi-attribute measurement of salmon meat quality in wild and farmed fish. There are vast amounts of benefits of Raman spectroscopy over the typical HPLC method, including quantitation of collagen and carotenoids as well as fat content, quick analysis in the range of just a few minutes for each sample, and no requirement for destructive or arduous sample preparation. In the future, studies on model development will allow for fast implementation in processing plants.
References
- Afseth, N. K.; Wold, J. P.; Segtnan, V. H. The Potential of Raman Spectroscopy for Characterisation of the Fatty Acid Unsaturation of Salmon. Anal. Chim. Acta 2006, 572 (1), 85–92.
- Alfnes, F.; Guttormsen, A. G.; Steine, G.; Kolstad, K. Consumers’ Willingness to Pay for the Color of Salmon: A Choice Experiment with Real Economic Incentives. Am. J. Agric. Econ. 2006, 88 (4), 1050–1061.
- Moreno, H. M.; Montero, M. P.; Gómez-Guillén, M. C.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen Characteristics of Farmed Atlantic Salmon with Firm and Soft Fillet Texture. Food Chem. 2012, 134 (2), 678–685.
About Kaiser Optical Systems, Inc.
Kaiser Optical Systems, Inc. (Kaiser), an Endress+Hauser company, is the global leader in Raman spectroscopic instrumentation for laboratory, process, and manufacturing environments. Our solutions harness the powerful analytical information of Raman Spectroscopy to help our customers understand, measure, and control their chemistries.
As a trusted partner in Raman for over 30 years, Kaiser has a long history in production, including GMP manufacturing, with many proven successes. Our unmatched expertise, high quality solutions, and exceptional customer service sets Kaiser far above any other Raman option in the marketplace. Kaiser Raman technology is currently used throughout the chemical, food and beverage, oil and gas, pharmaceutical, and biopharmaceutical industries to optimize process efficiency and deliver quality products. Kaiser’s manufacturing and headquarters facility is in Ann Arbor, Michigan
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.