Since the 18th-century researchers have studied proteins1, but a number of discoveries in the 1960s that demonstrated sodium dodecyl sulfate (SDS) could facilitate the separation of proteins, ushered in a new era of protein science.
Now, researchers combine SDS with reducing reagents to separate purified multimeric complexes2 or they could examine complex mixtures of proteins. In the late 1960s and early 1970s, the introduction of the polyacrylamide slab gel permitted the simultaneous comparison of multiple samples3,4.
Add an improved solubilization buffer5 and that is present-day SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Though there are many elaborations of traditional 1D SDS-PAGE, reliable documentation for analysis is a common requirement and this requires high-quality precision instrumentation. Quality and precision are mainstays of Analytik Jena’s 50-year imaging history.
Well-suited for any laboratory, the UVP GelSolo is an entry-level imaging system that supplies exceptional documentation of protein gels and nucleic acid, to name a few.
The UVP GelSolo can capture the finest details in your samples as it is equipped with a 5 megapixel CMOS camera. A brief overview of 1D SDS-PAGE is provided below, together with some expert tips to get the most out of 1D SDS-PAGE gels.
Theory and practice: performing 1D SDS-PAGE
Electric field
Proteins do not have uniform charges across the polypeptide, unlike DNA molecules. Instead, proteins are made up of nonpolar, polar, weakly polar, and charged side chains or R-groups.
This array of electrical potential under native conditions causes proteins to migrate unpredictably and so mass cannot be determined accurately unless combined with isoelectric focusing as in 2D gel electrophoresis, but this technique is challenging, time-consuming, and unnecessary for most applications.
Fortunately, proteins are denatured and a uniform negative charge is imposed on the polypeptide by incorporating SDS, masking the intrinsic charges of the R-groups. Protein charge will approximate protein size as SDS binds near uniformly (1.4 g SDS/1 g protein).
Proteins are loaded onto a gel matrix and an electrical field is applied similar to DNA gel electrophoresis. According to their molecular weight, negatively charged polypeptides will migrate toward a positive electrode (see Table 1).
Table 1. Resolving layer acrylamide percentage and recommended protein size ranges.
| Concentration of acrylamide (%) |
Protein size (kDa) |
5
7.5
10
15 |
36-200
24-200
14-200
14-60 |
Voltages will change Depending on the type of gel that is employed during SDS-PAGE. Generally, users run protein samples for approximately 30 minutes at a low voltage if stacking and resolving layer are utilized, this is done to improve stacking and, therefore, band resolution.
Next, the voltage is increased and runs for up to two hours. Alternatively, manufactured precast gels allow users to run gels at higher constant voltages with run times as short as 30 minutes.
Gel matrix and gel casting
Traditional homemade gels are described here. As the name suggests, protein gels are composed of polyacrylamide. Bis-acrylamide forms a polymer in the presence of cross-linking reagents and buffers and quickly solidifies at room temperature.
The sieving properties of the gel can be augmented as in DNA gel electrophoresis, by altering the concentration of acrylamide. Gels usually contain a stacking and resolving layer. The region of the gel where the bands separate from each other is known as the resolving layer.
In order to retard the migration of the polypeptides this region has a higher concentration of acrylamide. On the other hand, the stacking layer has a lower concentration and promotes the stacking of the protein sample at the interface between the resolving and stacking layers.
First, the resolving gel is poured between two slabs of glass in a gel casting tray. The resolving layer is overlaid with 100 % ethanol or 2-propanol to promote a flat stacking/resolving interface.
The alcohol is washed out repeatedly with water after the resolving layer has polymerized, and the stacking gel is poured on top of the resolving gel. A comb is added as in DNA gel electrophoresis.
The gel is ready for the addition of protein samples once the stacking layer has polymerized. Alternatively, users can purchase precast gels, which ensure the reproducibility of experimental results.
Electrophoresis buffers
There are multiple buffers for SDS-PAGE. As with DNA gel electrophoresis, buffers are needed to conduct current during electrophoresis. Unlike DNA gel electrophoresis, discontinuous buffer systems are more common in SDS-PAGE.
For instance, a gel may contain Tris-HCl pH 8.8 in the resolving layer, Tris-HCl pH 6.8 in the stacking layer, and Tris-Glycine buffer pH 8.3 in the tank. Glycine can take on two charge states in this system.
Positively charged glycine in the tank buffer (pH 8.3) enters the stacking gel (pH 6.8) during electrophoresis and bears a neutral charge, so it moves slowly in the polar field created by the electrodes.
On the other hand, chlorine in the gel buffer migrates much quicker and creates a negative charge front ahead of the glycine. These opposing fronts bookend the protein samples, pinching them into a thin line as they migrate toward the stacking/resolving layer interface.
The charge state of glycine switches (pH 8.3) back to negative at the interface and its role is done. The proteins enter the resolving layer as a tight front, improving resolution, and synchronizing migration through the gel.
Visualization of proteins
As with DNA gels, there are a number of dyes utilized to label proteins in gels, arguably the most popular stain is Coomassie stain. The simplest Coomassie stain recipe requires brilliant blue R250 dye at a final concentration of 0.008 %, HCl at final concentration of 35-50 mM.
Gels may be stained with the dye for up to 3 hours and destained with water up to a few days. More commonly, researchers use 50 % methanol, 0.1 % brilliant blue R250, and 10 % acetic acid. Researchers are starting to employ less toxic techniques for protein analysis, similar to the movement away from ethidium bromide.
Imaging on the UVP gelSolo with the biotium 1kb DNA ladder
Sample preparation and running condition
In order to assist with protein gel images, the UVP GelSolo is equipped with epi-white light illumination. Furthermore, white light converter plates are available for the GelSolo as an accessory feature. Mouse serum samples were quantified using the Bradford Assay and measured on an Analytik Jena Specord 250 UV/Vis spectrophotometer.
Samples were serially diluted 1:2 and loaded onto a 4-12 % Bolt Bis-Tris Plus gel from Invitrogen (Waltham, MA). Total protein per lane ranges from 20 μg to 39 ng from left to right.
Samples were solubilized 1:2 in 2x Laemmli buffer from Bio-Rad (Hercules, CA) and boiled at 85 oC for 5 minutes before loading. Per the manufacturer’s instructions, the gel was run at 200 V (constant voltage) for 32 minutes.
In order to remove salt and excess detergents that may interfere with the protein stain, the gel was rinsed 3 x 5 minutes in milliQ water. The gel was stained for 1 hour with SimplyBlue Safe Stain from Invitrogen (Waltham, MA) and de-stained for 3 hours with milliQ water.
Image capture conditions
The UVP GelSolo was used to capture the gel image in Figure 1 and the exposure time was set to 22 ms. A UV transilluminator was utilized for excitation at 302 nm with a white light converter plate and the histogram adjustment was set to auto.
Although the maximum sensitivity protocol from the manufacturer was not used, using the basic protocol it was possible to reach near the limit of detection of the dye (i.e. 20 ng) since lane 10 represents a total of 39 ng with 3 visible bands. This sensitivity is a testament to the quality and robustness of Analytik Jena’s line of advanced imaging systems.
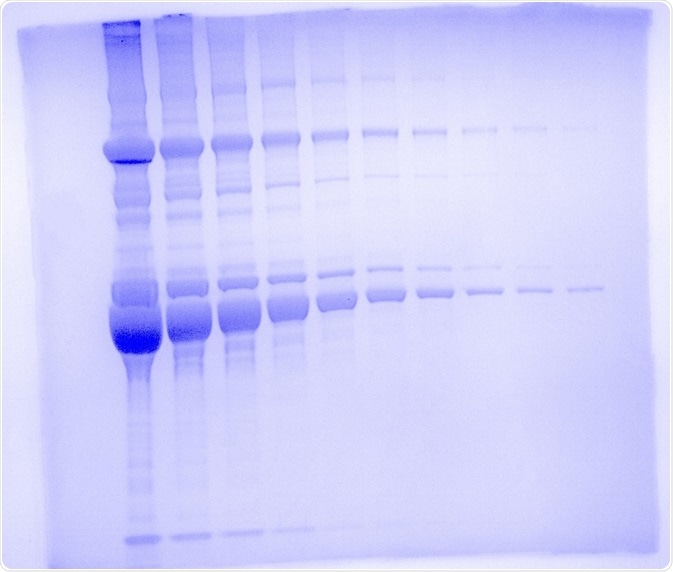
Figure 1. One dimensional SDS polyacrylamide gel electrophoresis using the UVP GelSolo. Mouse serum was run on the gel in 1:2 dilution series from 20 μg to 39 ng on a 4 - 12 % Bolt Bis-Tris Plus Gel for 32 minutes at 200 V. Gel was stained with SimplyBlue Safe Stain and imaged on the UVP GelSolo with 302 nm UV light and a white light converter plate with an exposure time of 22 s. The image was pseudocolored with Coomassie blue.
Expert tips
- Add the same volume of 1X Laemmli buffer to your samples and to your empty wells to avoid anomalous migration patterns.
- Clean outwells with running buffers prior to loading samples to promote even migration of samples through the gel.
- Cut costs by reusing the running buffer at least once, but pay attention to overheating.
- Use a lab marker to outline wells on the gel so that loading samples into wells is easier.
- Dispense sample into the well slowly so that samples aren’t accidentally forced out of the well.
- For extended electrophoresis runs, place the gel running tank in ice in a cold room to keep the buffer from overheating.
- Avoid loading samples or ladder in end wells to avoid edge effects.
- Do not overload wells. Do not exceed 20 μg for a complex mixture of protein or 2 μg for purified protein.
- Spin down samples after heating and vortexing, as some of the samples may evaporate and condense on the tube lid, therefore changing sample concentration.
- Vortex samples before and after heating to promote homogeneity of solubilization.
References and Further Reading
- Vickery, H. B. The Origin of the Word Protein. Yale J Biol Med 22, 387–393 (1950).
- Pederson, T. Turning a PAGE: the overnight sensation of SDS-polycrylamide gel electrophoresis. The FASEB Journal 22, 949–953 (2008).
- Reid, M. S. & Bieleski, R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Analytical Biochemistry 22, 374–381 (1968).
- Studier, F. W. Bacteriophage T7. Science 176, 367–376 (1972).
- Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680 (1970).
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About life science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About analytical instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About lab automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.