Zymo’s Quick DNA/RNA Viral MagBead Kit was automated using Analytik Jena’s Cybio FeliX pipetting robot.
The CyBio FeliX configuration, optimized for nucleic acid (NA) extraction, offers a convenient and user-friendly solution for sample processing in downstream applications, including polymerase chain reaction (PCR) analysis.
Zymo’s Quick DNA/RNA Viral MagBead Kit is highly adaptable and fully compatible with the NA-preserving DNA/RNA Shield, facilitating efficient sample storage. The CyBio FeliX system further streamlines laboratory workflows by automating component pre-filling and extraction through liquid handling automation. Its user-friendly CyBio FeliX Application Studio software ensures ease of use and maximizes the solution's efficiency.
Materials & methods
Samples
- Collect human saliva samples and swabs using a DNA/RNA shield
- Transport swabs in transport medium
- Use PBS as an extraction control
Instrumentation
- The CyBio FeliX eXtract hardware configuration consists of:
- CyBio FeliX Basic Unit with Enclosure (Cat.# OL5015-24-100, Analytik Jena)
- CyBio FeliX Extraction Set (Cat.# OL5015-25-120, Analytik Jena)
- LightCycler® 480 Instrument II, 384-well (Cat.# 05 015 243 001, Roche)
Consumables and chemical products
- Quick-DNA/RNA Viral MagBead (Cat.# R2141-E, Zymo Research).
- Zymo Research’s R1107-E DNA/RNA Shield Collection Tubes with Swab-DX
- DNA/RNA Shield (2X Concentrate, Cat.# R1200-25, Zymo Research)
- 2-Mercaptoethanol (Cat.# M3148, Millipore Sigma)
- Analytik Jena 845-FX-8500025, Deep Well Plate 96 square well, 2.0 mL
- Two-column reservoir
- Four-column reservoir
Sample preparation
- Add 500 µL 2-mercaptoethanol to 100 mL of viral DNA/RNA buffer (final concentration 0.5 % [v/v]).
- Add 80 mL of 99.5–100 % isopropanol to the MagBead DNA/RNA Wash 1 concentrate.
- Add 120 mL of 99.5–100 % isopropanol to the MagBead DNA/RNA Wash 2 concentrate.
- Reconstituted lyophilized Proteinase K to 7.7 mg/mL in Proteinase K Storage Buffer via vortexing. Material not used immediately was frozen in aliquots.
Samples were either processed immediately after collection or held inactivated at room temperature for further examination. No additional treatment was required for samples stored in the DNA/RNA Shield medium. Other samples were inactivated using an equal volume of 2x concentrated DNA/RNA Shield.
- Samples in DNA/RNA Shield collection devices (swabs, saliva, etc.), Swabs (UTM/VTM, PBS, saline, etc.), and inactivated samples:
- Transfer 400 µL sample to Process Plate, then immediately purify it
- Combine 200 µL of probe with 200 µL of PBS, then immediately purify
- Liquids, including plasma, serum, cerebrospinal fluid (CSF), blood, saliva, urine, cell suspension, and culture media:
- Add an equal volume of 2x DNA/RNA Shield, then mix.
- Combine 100 µl of supernatant/swab with 100 µl of medium, 200 µl of shield, and 20 µl of Proteinase K.
- Transfer 400 µL sample to Process Plate, then proceed with purification.
Method
To extract NAs, the Analytik Jena CyBio FeliX eXtract setup was used. The configuration includes the necessary accessories (such as a 96-well plate shaker), allowing seamless implementation of the Zymo kit.
CyBio FeliX setup
The kit reagents can be prefilled manually or through a scripted process that allows for column-wise assay preparation.
This setup allows users to extract between eight and 96 samples in increments of eight. For manual preparation, fill the desired number of wells per plate with the kit reagents listed in Table 1. Complete prefilling of the plate is not required; the user may prepare as many reactions as needed.

Figure 1. Analytik Jena CyBio FeliX hardware configuration and decks equipped for extraction applications. Image Credit: Analytik Jena US
Table 1. Manual plate preparation. The indicated volumes of liquid are calculated volumes per well/extraction. Source: Analytik Jena US
| Plate |
Label |
Content per well |
| Plate 1 |
Process |
400 μL sample + 20 μL Proteinase K |
| Plate 2 |
Viral DNA/RNA Buffer + Mag Beads |
800 μL Viral DNA/RNA Buffer + 20 μL MagBinding Beads*
optional: PCR IC as recommended by the detection assay |
| Plate 3 |
MagBead DNA/RNA Wash 1 |
600 μL MagBead DNA/RNA Wash 1 |
| Plate 4 |
MagBead DNA/RNA Wash 2 |
600 μL MagBead DNA/RNA Wash 2 |
| Plate 5 |
Ethanol |
1100 μL Ethanol, 95-100 % |
| Plate 6 |
DNase/RNase-free Water |
250 μL RNase-free Water (for Elution) |
| Plate 7 |
Elution |
Empty |
*Vortex MagBinding Beads thoroughly prior to dispensing. MagBinding Beads settle quickly, ensure that beads are kept in suspension while dispensing.
For automatic prefilling, empty plates are placed on the CyBio FeliX deck (Figure 2, left), and liquid is transported from reservoir plates. The script/protocol is included in CyBio FeliX eXtract’s Application Studio, prompting the user to specify the quantity of samples.
As an additional checkpoint, once the deck positions are loaded according to the software instructions, the application displays the calculated minimum reagent amounts required in the reservoirs for the procedure.
The procedure execution time varies with the number of samples, ranging from seven minutes (eight samples) to 38 minutes (96 samples).
The deck structure for the primary extraction process is slightly different from the CyBio FeliX design used for automated prefilling (Figure 2, right). This allows the user to proceed quickly to the main extraction technique.
A message box displays the deck-loading instructions during automated prefilling to prepare the CyBio FeliX for extraction. The procedure time for a whole plate (96 samples) is 52 minutes.
In detail, the total process time amounts to:
Source: Analytik Jena US
| |
|
| Reservoir filling with kit solutions and deck loading: |
4 minutes |
| Automated prefilling (full plate): |
38 minutes |
| Deck preparation for extraction: |
2 minutes |
| Automated extraction (full plate): |
52 minutes |
| |
Total: 96 minutes (1 minute/sample)* |
*This calculation does not include the time required to transfer samples from primary tubes into the process plate, which can be carried out during the automated plate prefilling.
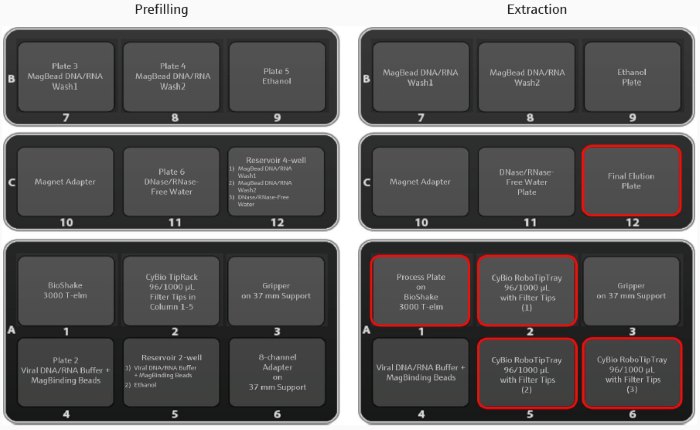
Figure 2. Deck layouts for automated prefilling (left) and the main extraction protocol (right). Image Credit: Analytik Jena US
Most of the deck arrangement is preserved between protocols, allowing for smooth operation. Changed deck positions are indicated in red. Prefilled plates remain in place, but two reservoirs (5, 12), a TipRack (2), and an 8-channel adaptor (6) are replaced with RoboTipTrays (2, 5, 6) and the final elution plate (12).
Extraction can begin immediately after placing the sample-loaded process plate directly on the BioShake (1).
To minimize the impact of residual ethanol on PCR efficiency, a higher elution volume (100 µL) was chosen over the manual’s recommendation due to the automated setup’s restricted evaporation capabilities.
For comparison, a sample subset was eluted with 50 µL to examine PCR inhibition versus potentially enhanced concentration obtained with a lower elution volume. Extracted samples can be detected immediately or frozen for later processing.
Polymerase chain reaction
A customized PCR system was employed to amplify viral target sequences, as described in the Drosten protocol published in January 2020.1
The assay included three SARS-CoV-2 targets (RdRP gene, E gene, and 2019-nCoV specific RdRP gene), and two controls: RPP30 (human sampling control) and extraction control.
As indicated in the extraction technique described above, 2.5 µL of extracted NA was utilized as a template. PCR samples were duplicated in 384-well PCR plates. Table 2 highlights the primers and probes used following a WHO-published methodology.2
Table 2. Primers targeting the E gene and a generic as well as a 2019-nCoV (SARS-CoV-2)-specific RdRP gene were used. Source: Analytik Jena US
| Assay/use |
Oligonucleotide |
Sequence 5‘-3‘ª |
Final concentration [nM] |
| RdRP |
gene RdRp_SARSr-F2 |
GTGARATGGTCATGTGTGGCGG |
600 |
| RdRP_SARSr-P1 |
FAM-CCAGGTGGWACRTCATCMGGTGATGC-BBQ |
200 |
| RdRp_SARSr-R1 |
CARATGTTAAASACACTATTAGCATA |
800 |
| E gene |
E_Sarbeco_F1 |
ACAGGTACGTTAATAGTTAATAGCGT |
400 |
| E_Sarbeco_P1 |
FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ |
200 |
| E_Sarbeco_R2 |
ATATTGCAGCAGTACGCACACA |
400 |
| RdRP gene specific for 2019-nCoV |
RdRp_SARSr-F2 |
GTGARATGGTCATGTGTGGCGG |
600 |
| RdRP_SARSr-P2 |
FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ |
200 |
| RdRp_SARSr-R1 |
CARATGTTAAASACACTATTAGCATA |
500 |
ª W is A/T; R is G/A; M is A/C; S is G/C. FAM: 6-carboxyfluorescein; BBQ: blackberry quencher
Results and discussion
Seven patient-derived samples were extracted using the Zymo Quick-DNA/RNA Viral MagBead Kit with an elution volume of 100 µL. Three samples were also generated with a reduced elution amount of 50 µL.
Table 3 displays the Ct values for the three viral targets (RdRP gene, E gene, 2019-nCoV), the extraction control, and the human control target RPP30, respectively. Within a sample, Ct values differed modestly among the viral SARS-CoV-2 targets.
The E gene and 2019-nCoV had similar Ct ranges (average intra-sample difference 0.94 ± 2.36 cycles), although RdRP had a higher average Ct than the E gene (1.17 ± 0.09 cycles). The constant amplification of the human RPP30 control confirmed proper sampling.
The extraction control results were stable, with only slight variation in Ct between samples (28.07 ± 0.19 cycles), including the PBS no template control (Sample D); this indicates a stable and reproducible extraction of NAs using the Zymo kit in combination with automated liquid handling on the CyBio FeliX system.
All except sample H tested positive for SARS-CoV-2. Sample H tested positive for coronavirus sequences but not the new SARS-CoV-2 virus.
The 2019-nCoV target was amplified in only one of the two replicates in sample E. Sample D is a no-template control in which PBS was extracted and PCR was negative except for the extraction control.
Table 3. PCR results of human samples extracted with the Zymo Quick-DNA/RNA Viral MagBead Kit and analyzed for the viral targets E, RdRP and 2019-nCoV, the human control target RPP30, and the extraction control. Samples were analyzed by PCR in duplicate, except for sample H. Source: Analytik Jena US
| Sample |
E gene |
RdRP gene |
2019-nCoV |
RPP30 |
Ext. control |
| A |
23.41 ± 0.17 |
24.60 ± 0.13 |
26.89 ± 0.15 |
34.01 ± 0.24 |
27.90 ± 0.00 |
| B |
26.04 ± 0.09 |
27.28 ± 0.17 |
29.21 ± 0.03 |
35.94 ± 0.48 |
28.08 ± 0.05 |
| C |
29.92 ± 0.22 |
31.01 ± 0.28 |
29.49 ± 0.00 |
31.26 ± 0.08 |
27.88 ± 0.08 |
| E |
32.94 ± 0.2 |
34.03 ± 0.82 |
29.45 |
31.34 ± 0.22 |
27.94 ± 0.04 |
| F |
28.81 ± 0.2 |
30.12 ± 0.17 |
30.01 ± 0.15 |
31.63 ± 0.09 |
28.27 ± 0.23 |
| G |
28.18 ± 0.05 |
29.44 ± 0.08 |
29.87 ± 0.13 |
35.16 ± 0.00 |
28.19 ± 0.02 |
| H |
33.53 |
34.59 |
no Ct |
29.11 |
27.85 |
| D (PBS) |
no Ct |
no Ct |
no Ct |
no Ct |
28.42 ± 0.21 |
The automated extraction used a 50 µL elution volume instead of the typical 100 µL. Three of the seven samples (B, F, and G) were evaluated, and the average Ct was marginally but consistently higher when utilizing the lower elution volume (plus 1.17 ± 0.84 cycles).
Differences tended to be target-specific, with the SARSCoV-2-specific 2019-nCoV being the most affected, with a difference of more than two cycles (Table 3).
The results suggest that elution inhibition caused by residual washing ethanol had a greater impact on NA concentration in the eluates than the concentration effect of using a smaller elution volume. Consequently, a 100 µL elution volume was established as the standard.
The Ct values of three samples were evaluated with PCR after being eluted with two different volumes (50 µL and 100 µL). Except for the extraction control, which yielded contradictory findings, all target amplifications utilizing lower elution volumes were more effective. As a result, 100 µL was set as the standard protocol.
Table 4. Effect of elution volume on PCR results. Source: Analytik Jena US
| Sample / elution volume |
PCR target |
| E gene |
RdRP gene |
2019-nCoV |
RPP30 |
Ext. control |
| B (50 μL) |
26.60 |
27.80 |
31.75 |
37.66 |
27.53 |
| B (100 μL) |
26.04 |
27.28 |
29.21 |
35.94 |
28.08 |
| F (50 μL) |
29.77 |
30.80 |
32.10 |
33.70 |
27.49 |
| F (100 μL) |
28.81 |
30.12 |
30.01 |
31.63 |
28.27 |
| G (50 μL) |
29.16 |
30.00 |
31.88 |
no Ct |
30.44 |
| G (100 μL) |
28.18 |
29.44 |
29.87 |
35.16 |
28.19 |
Overall, the results were consistent with those obtained using a commercial magnetic bead-based kit for viral NA extraction, previously automated on the CyBio FeliX platform.
Using the same sample input and elution volume for extraction, PCR analysis of samples from the Zymo Quick-DNA/RNA Viral MagBead extraction produced Ct values that were 1.6 ± 0.6 cycles lower than those obtained with the comparative method (data not shown).
Summary
This article demonstrates how to automate NA extraction using Analytik Jena’s CyBio FeliX and Zymo’s QuickDNA/RNA Viral MagBead kit for downstream PCR analysis.
This configuration combines the flexibility and compatibility of Zymo’s kit, including its use of DNA/RNA Shield for sample preservation, with the automated liquid handling capabilities of the CyBio FeliX system.
The automated approach involves prefilling components and performing the entire extraction process, optimized through the CyBio FeliX Application Studio software. By automating the NA extraction procedure with the Zymo kit, the CyBio FeliX significantly reduces hands-on time.
The automated extraction process takes around 52 minutes for a plate containing 96 samples. PCR is used alongside specialized setups to amplify viral target sequences. The results revealed consistent amplification of viral targets, human samples, and extraction controls.
The Zymo Quick-DNA/RNA Viral MagBead extraction performs similarly but with lower Ct values compared to another kit. The automated approach provides effective and user-friendly NA extraction from various sample types, aided by a combination of Analytik Jena hardware and software and the Zymo kit.

Figure 3. CyBio FeliX. Image Credit: Analytik Jena US
Recommended device configuration
Table 5. Device and accessories. Source: Analytik Jena US
| Article |
Article number |
| CyBio FeliX Basic Unit with Enclosure |
OL5015-24-100 |
| CyBio FeliX Extraction Set |
OL5015-25-120 |
| CyBio TipRack 96/1000 μL |
OL3811-25-939-F |
Acknowledgments
The authors extend their gratitude to Ilva Leckebusch, B.Sc., for her invaluable contribution.
References
- Corman, Victor M et al. “Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.” Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin vol. 25,3 (2020): 2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045
- https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About Life Science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results. This will surely ease your daily work and speed up your work processes in a certain way.
All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About Analytical Instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems that date back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters, and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About Lab Automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high-quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness, and simplicity.
Moreover, the Automation Team designs produce, and install fully automated systems tailored to our clients' application, throughput, and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems, we handle your compounds, biomolecules, and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.