Jan 19 2021
It has been one year since the outbreak of COVID-19, but there is still an increasing number of confirmed cases. The epidemic situation is still challenged all over the world. The effective drug remains to be the key factor for keeping the epidemic in check and reducing mortality.
At present, the most effective treatment for COVID-19 is the plasma from convalescent patients, and its active ingredient is the neutralizing antibodies. The much anticipated COVID-19 vaccines also depend on the neutralizing antibodies produced by vaccines to achieve the protective effect.
The antiviral mechanism of SARS-CoV-2 neutralizing antibody
The COVID-19 pathogen SARS-CoV-2 binds with the receptor angiotensin-converting enzyme (ACE2) on human cells through the Spike protein (S protein) to undergo a cell membrane infusion process, allowing for the entry of the virus into the cells through endocytosis for replication, processing, and infecting the body.
The blocking neutralizing antibody against SARS-CoV-2 S protein RBD can prevent the binding of S protein RBD to ACE2, thereby preventing the virus from invading host cells and ultimately preventing virus infection. This is an important pathway for neutralizing antibodies to exert their antiviral effects (Figure 1).
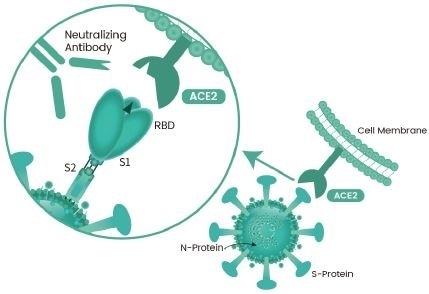
Figure 1. The mechanism of action of SARS-CoV-2 RBD neutralizing antibody. Image Credit: Sino Biological Inc.
Overview of global SARS-CoV-2 neutralizing antibody R&D
On April 28, 2020, the Ministry of Science and Technology of the People's Republic of China mentioned in the Guidance for Emergency Project Application for R&D of SARS-CoV-2 Neutralizing Antibody Products that, neutralizing antibodies have the potential to block the virus from infecting target cells, and monoclonal antibodies have the advantages of the definite mechanism of action and convenient access to mass production, making it the vital research area of anti-SARS-CoV-2 medicines. In May 2020, the team against COVID-19 involving 12 top scientists in the United States issued a proposal for fighting against COVID-19, which also believed that monoclonal antibody drugs are considered a novel and potential antiviral alternative option.
At present, SARS-CoV-2 neutralizing antibody drugs developed by many global enterprises and research institutions have entered the clinical trials around the world, and examples include the monoclonal neutralizing antibody produced by U.S. Regeneron and Vir Biotechnology; recombinant anti-nCoV Humanized Monoclonal Antibody Solution for Injection JS016 jointly developed by Junshi Biosciences and Institute of Microbiology, Chinese Academy of Sciences, etc.; SARS-CoV-2 neutralizing antibody BI 767551 produced by UKK, UMR, DZIF, and Boehringer Ingelheim; and high-affinity SARS-CoV-2 neutralizing antibody BD-368-2 developed by the team of Peking University led by Academician Xiaoliang Xie. Most of these neutralizing antibodies are derived from the plasma of convalescent patients or the plasma of animals immunized with SARS-CoV-2 antigen.
There are numerous varieties of antibodies in the plasma, reaching as high as about 107-108. Therefore, it is extremely difficult to find SARS-CoV-2 neutralizing antibodies in the plasma. However, high-throughput single-cell sequencing can significantly shorten the antibody screening process, from the previous several years to the current several months. The team led by Academician Xiaoliang Xie isolated a single B cell that can secrete SARS-CoV-2 antibodies from thousands of B cells in the blood of convalescent patients using the high-throughput single-cell sequencing and identified the memory B cells that generated neutralizing antibody by determining the transcriptome of each cell. Then, the enriched memory B cells were screened to obtain the antibody-related gene sequences, and the antibody is expressed in vitro using genetic engineering technology. Sino Biological Inc. provided services of rapid high-throughput antibody expression and screening for the SARS-CoV-2 monoclonal antibody research and development project undertaken by the team of Peking University led by Academician Xiaoliang Xie.
The SARS-CoV-2 neutralizing antibody R&D of Sino Biological Inc
In addition, based on the experienced monoclonal antibody technology platforms: single B cell technology, phage library technology, mouse hybridoma technology (Figure 2 and Figure 3), and the recombinant protein expression platforms, the R&D group of Sino Biological Inc. chose the self-developed active recombinant Spike protein as the antigen to obtain many anti-RBD Mab Clones. First, an ELISA competitive neutralization screening platform was used to quickly screen the monoclonal antibody candidate strains that can block the binding of RBD and ACE2 protein, and then SARS-CoV-2 pseudovirus neutralization screening platform (Figure 4) was used to screen neutralizing functional antibodies that effectively block the invasion process. Presently, many high-affinity, high-specificity rabbit monoclonal and mouse monoclonal neutralizing antibodies have been successfully obtained (Figure 5) and applied in the SARS-CoV-2 studies.
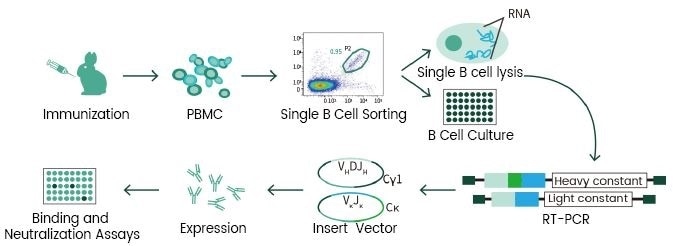
Figure 2. Single B cell technology. Image Credit: Sino Biological Inc.
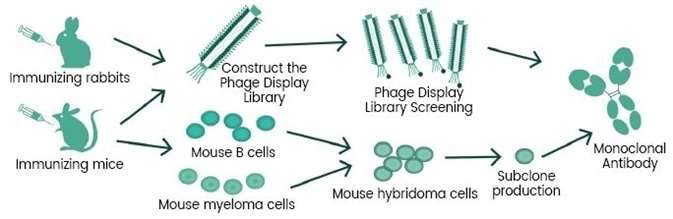
Figure 3. Phage library technology & hybridoma technology. Image Credit: Sino Biological Inc.
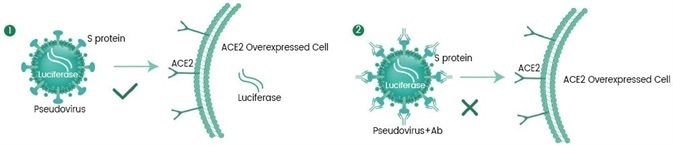
Figure 4. SARS-CoV-2 pseudovirus neutralization screening platform. Image Credit: Sino Biological Inc.
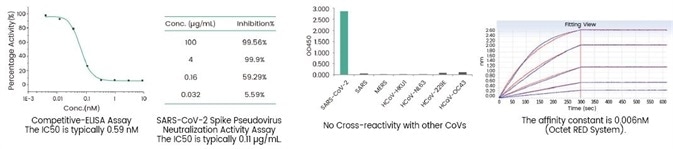
Figure 5. SARS-CoV-2 neutralizing antibody, rabbit MAb (Sino Biological Inc. Cat# 40592-R001). Image Credit: Sino Biological Inc.
The challenges in the R&D of SARS-CoV-2 neutralizing antibody
SARS-CoV-2 is a single-stranded RNA-enveloped virus. Studies have confirmed that the mutation frequency of RNA viruses is higher than that of the DNA viruses. The intervention of the SARS-CoV-2 in the human immune system and its rapid global spread provide favorable opportunities for the mutation of SARS-CoV-2. While the amino acid mutations may change the functions of the virus and its interactions with the neutralizing antibodies. Spike protein, as a key target for the SARS-CoV-2 to recognize receptors on the host cell surface and mediate cell fusion, has a higher mutation frequency, especially in the RBD region. So far, hundreds of mutants of S protein have been reported. An article titled "The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity" published in the journal of Cell found that different mutations have varied sensitivity to the neutralizing antibodies. Sino Biological used self-developed neutralizing antibodies and mutant proteins to perform studies and found that different neutralizing antibodies show different abilities in recognizing non-mutant proteins and RBD (V367F) mutant proteins. Moreover, the neutralizing antibodies have different abilities in neutralizing non- mutant proteins and S1 D614G mutant proteins (Figure 6). The result showed that frequent mutations are a major challenge to the protective ability to neutralizing antibodies.
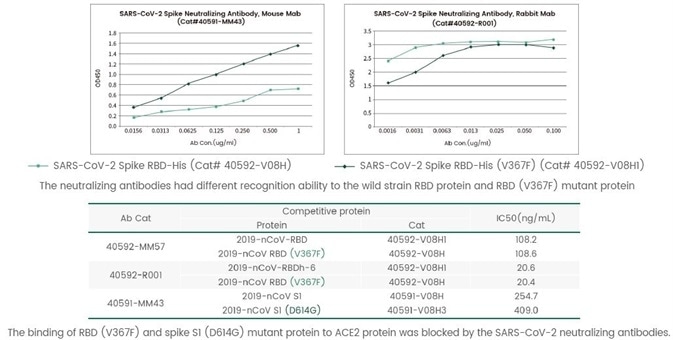
Figure 6. The recognition of SARS-CoV-2 neutralizing antibody to non-mutant and mutant proteins and the competitive neutralization ability by ELISA. Image Credit: Sino Biological Inc.
Although antibody screening specific for RBD and ACE2 binding epitopes is considered as the core for the development of SARS-CoV-2 neutralizing antibody, the mutations in the RBD region may allow newly generated strains to escape from the known neutralization effects of the RBD antibodies. Therefore, the development of neutralizing antibody combinations against different epitopes of RBD or the development of antibodies with high neutralizing activity against conserved epitopes will be effective strategies to prevent immune escape caused by viral mutations. Previously, many monoclonal antibodies with neutralizing activity against the S2 region, especially HR1 and HR2, have been successfully isolated in the SARS-CoV study. The NTD neutralizing antibody is also obtained successfully in the MERS-CoV study. In May 2020, Academician Chen Wei collaborated with the Zhou Qiang laboratory team of Westlake University, to isolate antibody 4A8 from the plasmocytes in the plasma of COVID-19 convalescent patients, which could recognize the fragile epitope of Spike NTD. Although it could not block the binding of S protein to ACE2, it could neutralize true viruses and pseudoviruses, indicating that it possesses a mechanism of virus neutralization independent of receptor binding inhibition.
In addition to studies on neutralizing antibodies targeting different targets, some researchers have investigated SARS-CoV-2 neutralizing antibodies made from different methods, for example, the 47D11 monoclonal antibodies found in humanized mouse experiments that can neutralize both SARS-CoV-2 and SARS, and nanobodies H11-D4 and H11-H4 derived from alpaca.
At present, clinical trials involving SARS-CoV-2 neutralizing antibodies are being actively carried out across the world. Neutralizing antibodies may become a specific medicine against SARS-CoV-2 and will forge double defensive lines of treatment and protection together with the use of vaccines, to safeguard the health of mankind.
About Sino Biological Inc.
Sino Biological, Inc. is a global leader in manufacturing affordable, high-quality reagents, including recombinant proteins, antibodies and cDNA clones—all in-house. We provide a one-stop shop for researchers and pharmaceutical companies around the world, helping our customers obtain the best reagents in the needed amounts to accelerate the pace of discovery and improve human health.

Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.