An invaluable tool for analyzing liposomes is Small-Angle X-ray Scattering (SAXS). Liposomes, a class of nanoparticles, have a phospholipid bilayer wall by which they are characterized.
They are attractive carriers for active ingredients in food, cosmetic and pharmaceutical applications because of their biocompatibility, ease of manufacturing and versatility. This means that liposomes are one of the few classes of nano-based drug delivery systems.
SAXS is an efficient tool for the incorporation of active components into the membrane, as well as probing the size, shape and flexibility of the membrane. SAXS can also give some unique insights into the liposomes that are not accessible by other techniques and provides statistically relevant results.
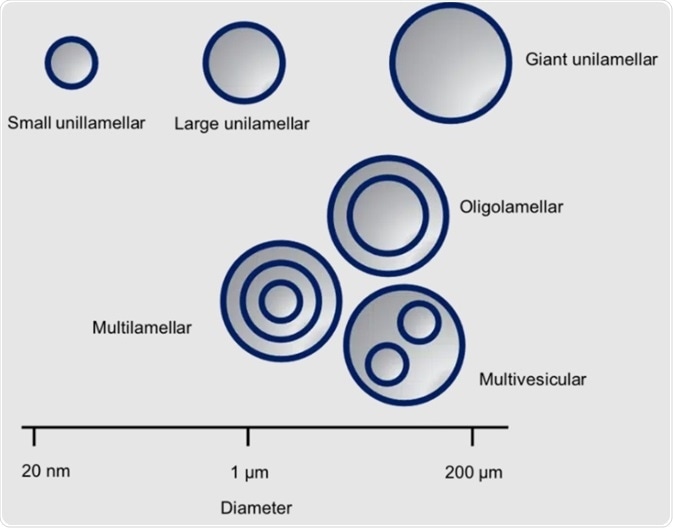
Image Credit: Xenocs
Liposomes were first described in the early 60s. They have lipid bilayer walls mainly consisting of phospholipids and are spherical vesicles. Their size can vary from between tens of nanometers to over 200 micrometers.
There are two types of liposomes: multilamellar and unilamellar. There are several categories of multilamellar vesicles: multivesicular, oligolamellar and multilamellar liposomes, as shown in the following figure.
They are relatively easy to manufacture and have good biocompatibility, making them ideal carriers in food2 and cosmetic1 formulations, as well as for active pharmaceutical ingredients (APIs).
Liposomes often contain cholesterol or other lipophilic molecules, aside from various types of phospholipids.
Additionally, it is possible to modify the surface using polyethylene glycol (PEG) or other carbohydrates, small molecules, proteins, polymers and other moieties to modify their solubility, stability or direct them to the tissues where they should unload their active cargo.
Their success as nano drug-delivery systems stems from this versatility, as evidenced by the liposomes that are currently on the market in clinical formulations.(i)
DepoDur®, LMX-4® and Doxil® are some examples of successfully marketed formulations. These registered medicines all have an active ingredient that is too lipophilic to be administered directly. This means that they are instead encapsulated in a liposomal carrier.
Characterizing lamellarity and lipid bilayer thickness with SAXS
The production of liposomes for the above applications is regulated under Good Manufacturing Practice (GMP) guidelines to maintain constant quality and to safeguard the health of consumers.
According to these guidelines, any changes in the manufacturing process must be evaluated, and processes must be clearly defined and controlled.(ii) Small-Angle X-ray Scattering is a well-suited tool for morphological analysis, which is crucial for quality control.
The amount of lamellar bilayers, also known as lamellarity, is one of the parameters that is marked as important by the Food and Drug Administration (FDA). The lamellarity is a major factor in a liposome’s ability to both contain and retain the drug substance.(iii)
SAXS can easily assess the number of lipid bilayers and their exact conformation. Information about Alkyl chain spacing can be obtained by coupling the measurement with Wide Angle X-ray Scattering (WAXS), allowing for the determination of ripple, lipid or gel phases.
A recent publication from scientists from the University of La Sapienza and Universita degli Studi dell Aquila in Italy used SAXS in combination with other characterization methods to study the loading of quinoline in liposomes composed of different lipids.3
While quinoline is a versatile and well-known compound, it has low solubility, which limits its impact for pharmacological applications; this can be overcome with its inclusion into liposomes.
WAXS and SAXS measurements for liposomes made of different phospholipids (DOPC, DPPC) and of quinoline-loaded multilamellar vesicles with different quinoline formulations are shown below.
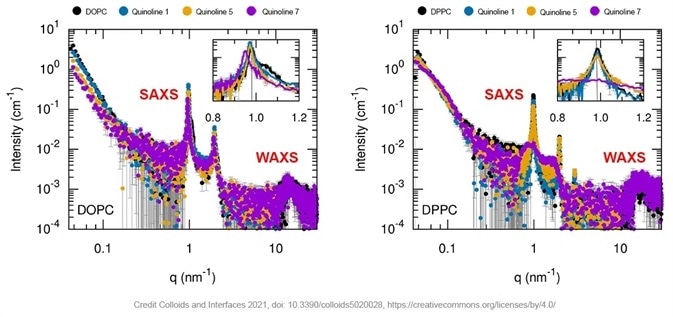
Figure 1. SAXS curves of empty and quinoline loaded phospholipid containing multilamellar vesicles (black curve is empty liposomes, orange, blue and purple curves are for different quinolines formulations). Insert is a zoom on the scattering peaks. Image Credit: Xenocs
The presence of vesicles with a desired degree of lamellarity is confirmed by well-defined, periodic scattering peaks. The exception to this is formed by the DPPC liposome loaded with quinoline7. The presence of liposomes with a much lower number of stacked bilayers is suggested by broad scattering peaks.
For characterizing the bilayer thickness, SAXS is the technique of choice because of its nanometer resolution and because it gives statistically relevant information from a ~ 1 cubic millimeter sample volume.
Additionally, it provides insight into the incorporation of active ingredients and membrane flexibility.1-2 The samples can be measured in the near-native state, accurately representing the natural state and enabling the measurement of samples that are difficult to freeze. This feature is unlike any other technique, including Cryo-Electron Microscopy.
Crucially, SAXS allows for the examination of the parameters listed above as a function of temperature and other environmental parameters.1,2,4-7
SAXS has additional advantages in some cases. For instance, currently, it is the only direct method for measuring the structure and dimension of PEG coronas of liposomes because this lacks sufficient contrast for other techniques like cryo-TEM.8-9
SAXS characterization of liposomes sizes
SAXS can be used for other functions besides studying bilayer morphology, including size characterization of liposomes between a few nanometers to a few hundred nanometers, depending on the instrument and type of liposome.
A recent publication from the Karlsruhe Institute of Technology (KIT) used SAXS to investigate the use of liposomes to stabilize fluorocarbons-based emulsions.10 This foundational research investigated phospholipids with various chain lengths and head sizes, as well as different concentrations of the phospholipids.
SAXS unambiguously confirmed the liposome formation, something that was not clear for freshly prepared samples from other measurement techniques. Additionally, SAXS was shown to be insensitive to gas bubbles that are introduced during production in the samples.
This means SAXS can provide more reliable measurements of the size of the liposomes than the intensity weighted mean hydrodynamic size as measured by photon correlated spectroscopy (PCS)-also known as dynamic light scattering (DLS).
For this experiment, PCS measurements were hindered by the small difference in the refractive indices of both phases; this issue is not a problem for SAXS because instead of relying on the refractive index, it relies on differences in electron density.
The image below shows the radially averaged scattering patterns of the liposomes at different concentrations (right) and with different phospholipids (left); this data can be modeled with high quality fits.
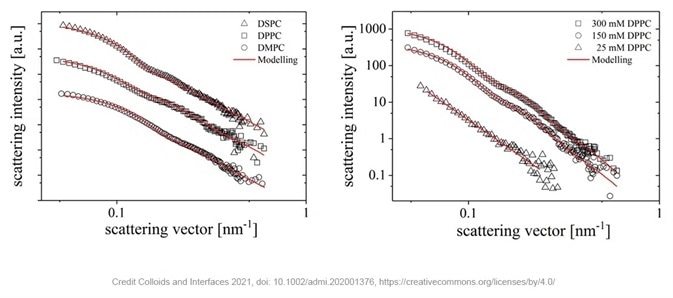
Figure 2. SAXS measurements of different liposomes consisting of phospholipids with different chain lengths (left) or prepared with different concentrations of DPPC in the stock suspension (right). Image Credit: Xenocs
The results indicate that the liposomes’ size increases with the increasing length of the phospholipid tail from 48 nm for DMPC to 59 nm for DSPC and 61 nm for DPPC. By varying the concentration of DPPC from 25 to 300 mM, liposomes from 61 nm to well over the detection limit of the instrument are formed.
It can be concluded from the increase in scattering intensity that the more phospholipids are present, the more liposomes can be formed.
The results from this experiment represent an important first step in the development of fluorocarbon-in-water emulsions for pharmaceutical use. Recently, there has been a growing interest in fluorocarbons as carriers for gaseous oxygen as well as for hydrophobic drugs.
Fluorocarbons can also be used as MRI contrast agents. The use of liposomes might be able to significantly improve the production process by reducing the waste of the active ingredients.
Xenocs SAXS solutions for R&D and manufacturing of drug carriers
Nanoparticles, including liposomes, are attractive vehicles for drug payload delivery as they provide endless possibilities for tackling various pharmaceutical needs. Xenocs provides solutions for the effective development of nanoparticles, including liposomes.
Xenocs’ X-ray scattering products provide reliable and robust results, thereby reducing the R&D phase. They are also well suited for quality control and manufacturing monitoring, ensuring that safe products reach the market faster.
References
- Sreij, R.; Dargel, C.; Moleiro, L. H.; Monroy, F.; Hellweg, T. Aescin Incorporation and Nanodomain Formation in DMPC Model Membranes. Langmuir 2017, 33 (43), 12351–12361. https://doi.org/10.1021/acs.langmuir.7b02933.
- Chaves, M. A.; Oseliero Filho, P. L.; Jange, C. G.; Sinigaglia-Coimbra, R.; Oliveira, C. L. P.; Pinho, S. C. Structural Characterization of Multilamellar Liposomes Coencapsulating Curcumin and Vitamin D3. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2018, 549, 112–121. https://doi.org/10.1016/j.colsurfa.2018.04.018.
- Battista, S.; Marsicano, V.; Arcadi, A.; Galantini, L.; Aschi, M.; Allegritti, E.; Del Giudice, A.; Giansanti, L. UV Properties and Loading into Liposomes of Quinoline Derivatives. Colloids and Interfaces 2021, 5 (2), 28. https://doi.org/10.3390/colloids5020028.
- Borro, B. C.; Toussaint, M. S.; Bucciarelli, S.; Malmsten, M. Effects of Charge Contrast and Composition on Microgel Formation and Interactions with Bacteria-Mimicking Liposomes. Biochimica et Biophysica Acta (BBA) – General Subjects 2021, 1865 (4), 129485. https://doi.org/10.1016/j.bbagen.2019.129485.
- Lim, S. W. Z.; Wong, Y. S.; Czarny, B.; Venkatraman, S. Microfluidic-Directed Self-Assembly of Liposomes: Role of Interdigitation. Journal of Colloid and Interface Science 2020, 578, 47–57. https://doi.org/10.1016/j.jcis.2020.05.114.
- Wood, I.; Albano, J. M. R.; Filho, P. L. O.; Couto, V. M.; de Farias, M. A.; Portugal, R. V.; de Paula, E.; Oliveira, C. L. P.; Pickholz, M. A Sumatriptan Coarse-Grained Model to Explore Different Environments: Interplay with Experimental Techniques. Eur Biophys J 2018, 47 (5), 561–571. https://doi.org/10.1007/s00249-018-1278-2.
- BioXolver application note ANBX06 Probing the effect of oxidation on model lipid bilayers.
- Schilt, Y.; Berman, T.; Wei, X.; Barenholz, Y.; Raviv, U. Using Solution X-Ray Scattering to Determine the High-Resolution Structure and Morphology of PEGylated Liposomal Doxorubicin Nanodrugs. Biochim Biophys Acta 2016, 1860 (1 Pt A), 108–119. https://doi.org/10.1016/j.bbagen.2015.09.012.
- Nordström, R.; Zhu, L.; Härmark, J.; Levi-Kalisman, Y.; Koren, E.; Barenholz, Y.; Levinton, G.; Shamrakov, D. Quantitative Cryo-TEM Reveals New Structural Details of Doxil-Like PEGylated Liposomal Doxorubicin Formulation. Pharmaceutics 2021, 13 (1), 123. https://doi.org/10.3390/pharmaceutics13010123.
- Ullmann, K.; Meier, M.; Benner, C.; Leneweit, G.; Nirschl, H. Water-in-Fluorocarbon Nanoemulsions Stabilized by Phospholipids and Characterized for Pharmaceutical Applications. Advanced Materials Interfaces 2021, 8 (1), 2001376. https://doi.org/10.1002/admi.202001376.
-
-
-
About Xenocs
Xenocs is a supplier of x-ray scattering equipments (SAXS/WAXS) for characterizing the nanostructure and morphology of materials at the nanoscale. Such equipments are used for research, development, and production of advanced materials in various domains such as nanoparticles & colloïds, polymer, food science, cosmetics or biostructural research.
Created in 2000 as a spin off from Institute Laue Langevin, the company started activity offering its customers innovative X-ray sources, optics and collimation solutions for X-ray characterization of nanomaterials and nanostructures.
Xenocs delivered its first x-ray scattering equipment in 2008, setting new standards for the possibilities of using SAXS in the laboratory for characterization at the nanoscale.
Working closely with both academic and corporate customers, Xenocs has continuously focused on providing value through performance and ease of use, while also creating a global sales and service organization.
In 2016, Xenocs bought Saxslab with operations in Denmark and in Massachusetts, making it the leading provider of SAXS equipments worldwide. Xenocs headquarters are located in France, and the company has subsidiaries in USA, Denmark and Singapore as well as a strong network of local contacts.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.