All plate-based assay formats, including cellular, biochemical, and bead-based, require optimization for the identification of appropriate conditions for robust performance in routine screening applications.
SPT Labtech has developed an innovative positive displacement dispensing instrument for the optimization of assays, which can be utilized with either 96, 384, or 1,536 well assay plate formats. The broad range of assay volumes is realized by the utilization of precise, non-contact, disposable syringes for dispensing liquids.
Initial work has already evidenced the applicability of this technology for biochemical assay optimization.
A frequent issue for automated dispensers is the risk of impacting the long-term health and viability of cells because of shearing and stress effects.
The study detailed in this article investigates the compatibility of the instrument for cell-based assays by directly comparing the performance with hand dispensing. Three commonly utilized cell types in screening were used, namely hepatic (HepG2), epithelial (A431), and neuronal (SH-SY5Y).
When dispensed by the dragonfly® discovery automated dispenser, these cell lines show normal cell proliferation, viability, and apoptotic responses upon activation with staurosporine. These data sets display how dragonfly discovery can be effectively used in cell-based assays.
Key benefits
Cells dispensed by the dragonfly discovery automated liquid dispenser exhibit comparable responses to cells dispensed by hand when looking at:
- Proliferation profiles
- Cell viability
- Apoptosis
The utility of dragonfly discovery has been shown to dispense commercially available immortalized cell lines while maintaining cell viability and function.
Materials and methods
Cell preparation and seeding
The following immortalized cell lines of different hosts and lineage were employed:
- HepG2 (Sigma # 85011430-1VL; hepatic)
- A431 (Sigma # 85090402-1VL; epithelial)
- SH-SY5Y (Sigma # 94030304-1VL; neuronal)
Each of these cell lines were grown in respective culture media and trypsinized. Following this, they were resuspended at 10,000 cells/mL in a suitable complete culture medium.
Cells were subsequently dispensed (50 μL per well) with either a hand-held repeating pipette or the dragonfly discovery automated liquid dispenser into 384-well tissue culture plates (Greiner #781091).
The outer two wells contained culture medium only to act as an evaporation barrier to prevent plate edge effects from interfering with assay performance.
The plates were incubated at a temperature of 37 °C and 5% CO2 until assay intervention was necessary, as explained in the following experimental details.

Figure 1. dragonfly® discovery automated liquid dispenser. Image Credit: SPT Labtech
Proliferation and cell health
A 5 μL mixture of propidium iodide (Invitrogen cat# P3566) diluted in phosphate-buffered saline (ThermoFisher cat# 14190-094) and Hoechst 33342 (Invitrogen cat# H3570) were added at specified time points to provide working concentrations of 10 μM of each nuclear stain.
Incubation of the plate then took place at 37 °C and 5% CO2 for one hour, followed by the plate being scanned using mirrorball®. Each well’s total cell number was calculated by dividing the total area of Hoechst staining by the area of a single Hoechst-stained nucleus.
The number of dead cells for each well was calculated by the total area of propidium iodide staining divided by the area of a single propodium iodide-stained nucleus.
Propidium iodide stains dead and dying cells as it cannot pass through the intact cell membrane of a healthy cell.
The percentage of viable cells per well is calculated based on the quantities of dead cells and total cells. Each data point represents six replicates within the experiment.
The data sets displayed are representative of three complete experimental repeats.
Caspase 3/7 activity
Following incubation overnight at 37 °C and 5% CO2, the assay wells received an addition of 5 μL of staurosporine (Sigma # S4400) per well to provide the necessary working concentration of the drug. The plate was subsequently incubated for 24 hours at 37 °C and 5% CO2.
Wells were each treated with 5 μL of a detection mixture that contained Hoechst 33342 (Invitrogen cat# H3570) and CellEvent Caspase-3/7 (Life Technologies # C10423) to provide working concentrations of 10 μM and 1 μM, respectively.
Following this, the plate was incubated at 37 °C and 5% CO2 for a total of 30 minutes and subsequently scanned using the mirrorball. The total number of cells per well was calculated using the Hoechst scan, while the total caspase-3/7 intensity was calculated using the green channel.
Data was normalized to provide a measure of caspase-3/7 activity per cell per assay well. Each data point represents six replicates within the experiment. The data sets displayed are representative of three complete experimental repeats.
Results
The dispensing technology of the dragonfly discovery
In each of the channels (up to 10), there is a tight-fitting piston that travels within a pipette barrel. When coupled to the instrument’s piston rod, the positive displacement syringe is formed.
The distance and rates of acceleration and deceleration of the piston control how and when liquid is ejected from the tip.
Although each channel is completely independent of the other channels, it is possible for them to all be operated simultaneously, providing rapid but highly flexible dispensing.
This facilitates complex combination gradients in high-density (up to 1,536-well) microplates and high-speed bulk filling of common reagents.

Image Credit: SPT Labtech
Cells dispensed by dragonfly discovery exhibit normal proliferation profiles
Each of the three cell lines (HepG2, A431, and SH-SY5Y) were dispensed into 384-well cell culture plates either using a hand pipette or the dragonfly discovery. Comparable rates of cellular proliferation were revealed for the two dispensing methods, as displayed in Figure 2.
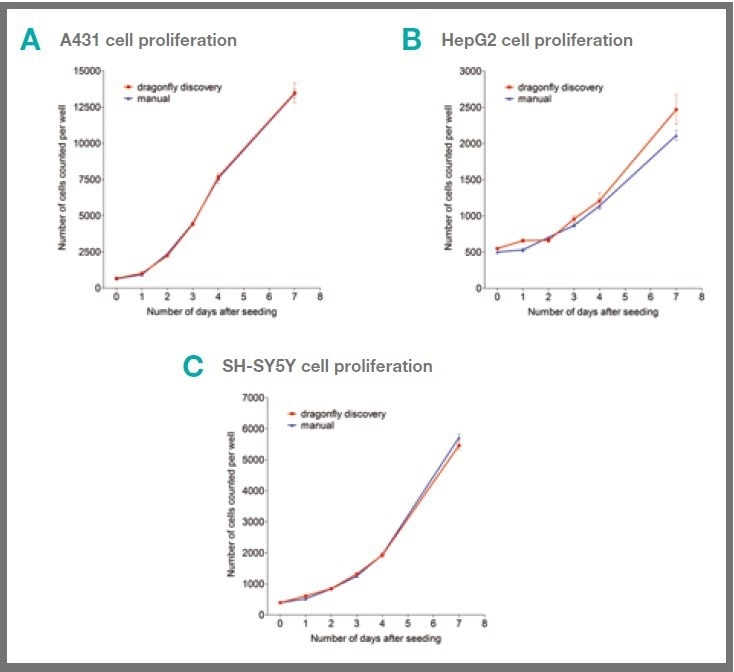
Figure 2. Cells dispensed by dragonfly discovery show similar proliferation to hand-dispensed cells. 50 μl of A431 (A), HepG2 (B) or SH-SY5Y (C) cells in complete culture medium were dispensed per well either by hand or by dragonfly discovery into 384 well cell culture plates. Cell proliferation was measured at indicated time points by the addition of Hoechst 33342 to give 10 μM working concentration. Cell number was determined by scanning on mirrorball. Each data point shows mean +/- s.e.m. of six replicate wells and data sets shown are representative of three separate experiments. Image Credit: SPT Labtech
Cells dispensed by dragonfly discovery exhibit normal viability rates
Following on from monitoring cellular proliferation, the next step was to investigate whether automated dispensing had any adverse effects on cell viability.
To this end, the three cell lines A431, HepG2 and SH-SY5Y were dispensed into 384 well cell culture plates either by hand pipette or by dragonfly discovery, and the relative levels of cell health were monitored.
Cells dispensed by dragonfly discovery showed similar viability to hand-dispensed cells (Fig 3).
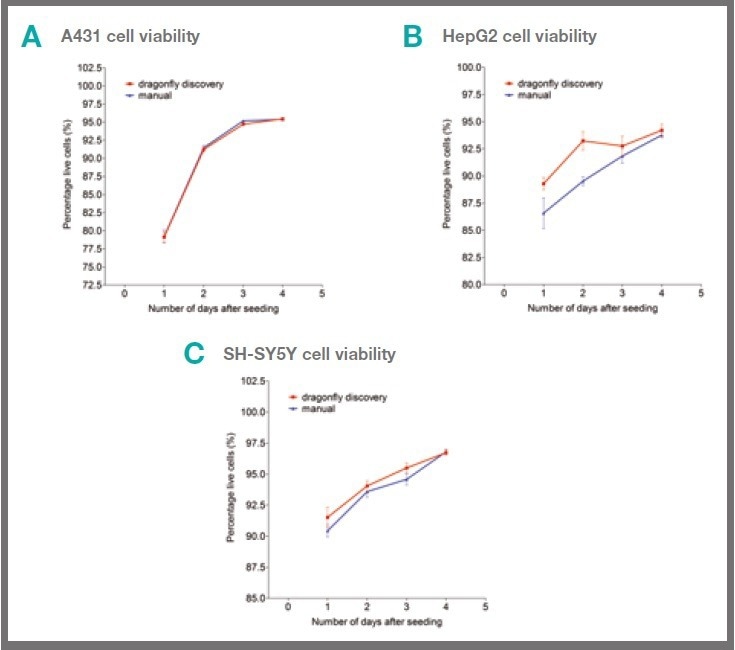
Figure 3. Cells dispensed by dragonfly discovery show similar viability to hand-dispensed cells. 50 μl of A431 (A), HepG2 (B) or SH-SY5Y (C) cells in complete culture medium were dispensed per well either by hand or by dragonfly discovery into 384 well cell culture plates. Cell viability was determined by the addition of Hoechst and Propidium iodide (giving 10 μM working concentration of each) to the wells at the indicated time point. Live/dead cell analysis was determined by scanning on mirrorball. Each data point shows mean +/- s.e.m. of six replicate wells and data sets shown are representative of three separate experiments. Image Credit: SPT Labtech
Cells dispensed by dragonfly discovery display normal drug-induced apoptotic response
Having shown that basic cell proliferation and viability were similar, the next step was to investigate whether cellular responses behaved as expected for cells dispensed by dragonfly discovery.
Dispensing cells by the dragonfly discovery itself does not lead to any significant activation of the caspase-3/7 apoptotic marker in A431 cells, as presented in Figure 4A. This was also the case with HepG2 and SHSY5Y cells (data not shown).
These cells exhibit a normal concentration-dependent activation of the caspase-3/7 marker following treatment with staurosporine, as shown in Figure 4B-D.
The lack of caspase-3/7 response by HepG2 cells to staurosporine treatment corresponds to the observations outlined in the literature,1 and the same lack of response is observed with HepG2 cells dispensed by both dragonfly discovery and by hand.
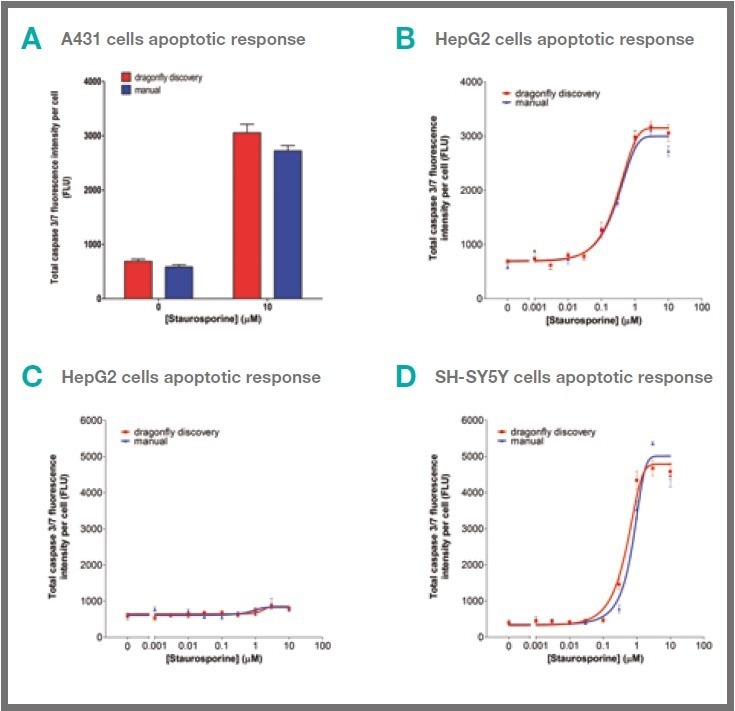
Figure 4. Cells dispensed by dragonfly discovery show similar apoptotic responses to hand‑dispensed cells. 50 μL of A431 (A & B), HepG2 (C) or SH-SY5Y (D) cells in complete culture medium were dispensed per well either by hand or by dragonfly discovery into 384 well cell culture plates. No apoptotic response was seen due to dispense in A431 cells (A). Cells were treated with staurosporine for 24 hours (B-D) before staining with Hoechst and CellEvent caspase‑3/7 reagent (giving 10 μM and 1 μM working concentrations respectively of each. Caspase-3/7 activity was determined by scanning on mirrorball. Each data point shows mean +/- s.e.m. of six replicate wells and data sets shown are representative of three separate experiments. Image Credit: SPT Labtech
Conclusions
The optimization of experimental conditions is a crucial step when setting up new assays to identify a robust protocol.
In cell-based experiments, the methods employed to dispense the cells must not impact cellular health or response to external stimuli, including potential drug candidates, as this compromises the reliability of the assay.
Comparisons were made of the cellular health and response to drugs of three frequently utilized cell lines when dispensed by hand or using the dragonfly discovery.
The results showed comparable rates of cellular proliferation for both dispensing methods, as displayed in Figure 2, which indicates that there are no negative impacts on basic cell function as a result of the automated dispense.
When cellular viability was monitored over several days, there was still no significant difference between the two methods of dispensing cells, as shown in Figure 3.
Since no detrimental effects arose regarding cell proliferation rates and viability, the cellular function was next to be investigated.
Apoptosis is a commonly studied process, and it is understood to be involved in the pathogenesis of many disease conditions, such as viral infections, AIDS (acquired immunodeficiency syndrome), cancer, autoimmune diseases, and neurodegenerative disorders.2
One marker for apoptosis is the activation of caspases 3 and 7. One concern regarding automated cell dispensing using a syringe with a diameter of 0.4 mm was that apoptosis may be induced due to exposure to shear forces.
However, these results demonstrate that the carefully controlled dragonfly-dispensed cells exhibit no difference in caspase-3/7 activity when compared to cells dispensed by hand, as shown in Figure 4A.
Comparable caspase-3/7 activation levels were revealed for both methods of dispensing cells following treatment with a concentration range of staurosporine (shown in Figure 4B-D).
In summary, this study has demonstrated that cells dispensed by the dragonfly discovery automated liquid dispenser display similar responses to those dispensed by hand regarding cell viability, proliferation profiles, and apoptosis.
The study also highlights the efficacy of the dragonfly discovery when it comes to dispensing commercially available immortalized cell lines whilst preserving cell function and viability.
References and further reading
- Staurosporine-induced apoptosis in Chang liver cells is associated with down-regulation of Bcl-2 and Bcl-XL. Michela Giuliano Giuseppe Bellavia Marianna Lauricella Antonella D’Anneo Barbara Vassallo Renza Vento Giovanni Tesoriere. International Journal of Molecular Medicine April 2004, 13(4), pp. 565-571. https://pubmed.ncbi.nlm.nih.gov/15010857
- Apoptosis in the pathogenesis and treatment of disease. Thompson CB. Science. 1995 Mar 10;267(5203):1456-62.
About SPT Labtech
We Design and Manufacture Robust, Reliable and Easy-to-Use Solutions for Life Science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the Heart of What We Do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A Problem-Solving State of Mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.