Researchers at the Institute for Genome Sciences, University of Maryland School of Medicine aim to conduct high-throughput culturomics analysis of the vaginal microbiome to develop live biotherapeutics that modulate the composition and function of the vaginal microbiome to improve women’s gynecological and obstetric health.
To achieve this, the team must first gain a more comprehensive understanding of the intra-species diversity within the beneficial bacterium, Lactobacillus crispatus. An in-depth within-sample cultivation is also necessary to acquire co-resident L. crispatus strains.
Classical culture techniques of plating and colony picking are time-consuming and laborious.
The team chose to utilize the dragonfly® discovery liquid handler from SPT Labtech to develop methods for high-throughput culturomics analyses, integrating bacterial sorting, single-cell dispensing, and dilution-to-extinction culture.
Hundreds or thousands of individual strains can be isolated into a single culture event by dispensing single cells into 384-well plates. Plated agar is then used to confirm that single-cell colony-forming units are being dispensed.
Dispensing individual cells into high-density wells guarantees the prevention of colony cross-contamination, enables robotics to resuspend the colonies for further culture and storage, and boosts the cultivation throughput.
For this method, 384-well plates need to be filled with an agar medium. However, molten agar dispensing is restricted by its narrow temperature range for dispensing (approximately 50-60 ̊ C) and viscosity (10-100 cps, 1.5 % sol, 60 ̊C), and manual pouring or pipetting is laborious and frequently results in bubble formation, making it tough to distinguish colonies.
Thus, for this study, dragonfly discovery was used for rapid, small-volume, molten agar dispensing into 384-well assay plates, followed by single-cell culture inoculation.
dragonfly discovery provides multi-channel, non-contact dispensing utilizing positive displacement syringes to guarantee accurate nanoliter to milliliter dispensing, regardless of liquid viscosity.

Image Credit: SPT Labtech
Methods
To prepare the agar medium, agar (RPI, #9002-18-0) was introduced to DifcoTMLactobacilli MRS Broth (BD, #288110) to a 1.5 % w/v solution and then autoclaved for 15 minutes at 121 ̊C, as per the manufacturer’s instruction.
The media-containing media bottle was subsequently positioned into a 65 ̊ C water bath near dragonfly discovery and allowed to equilibrate before dispensing.
A method was incorporated on the dragonfly discovery software to dispense molten MRS agar utilizing the “Corning 384 Low volume flat bottom (24x16)” plate file in a snaking pattern using ultra-low retention (ULR) syringes (SPT Labtech, #4150-07208).
Dispense settings were adjusted to a preset for “Gentle bulk addition above 5 μL,” setting the dispense speed at 3 mm/second.
The optimal syringe usage was determined by configuring the volumes dispensed in relation to the number of syringes used. This was crucial to prevent the agar from solidifying before the completion of the dispensing process.
Four syringes were placed at positions A2, A3, B2, and B3 to dispense 40 μL throughout one plate. For a dispense volume of 25 μL, six syringes were utilized at positions A2-A4 and B2-B4. This enabled two plates to be dispensed simultaneously with multi-aspiration mode.
Dispense times for the 25 μL and 40 μL protocols were 35 seconds and 1 minute 45 seconds per plate, respectively.
To optimize agar solidification time, syringes were engaged, standard reservoirs were added, and a 384-well assay plate (Corning, #3680) was positioned in the tray with the lid removed before initiating the instrument protocol. A serological pipette was employed to rapidly transfer molten MRS agar and aliquot suitable volume to every reservoir.
The protocol started immediately after the filling of the last reservoir. The process was repeated with fresh syringes and reservoirs for every aspiration. Plates were cooled in a laminar flow hood with the lid removed to avoid condensation.
Agar depth, with 25 μL and 40 μL dispense volumes, was measured at 4.8 mm and 9.5 mm, respectively (as shown in Table 1).
Table 1. Fill metrics and observed growth in 384-well agar-filled plates. Source: SPT Labtech
| Dispense volume (μL) |
Agar depth (mm) |
% Growth wells |
Lambda (λ)* |
| 25 |
4.8 |
25.3 |
0.3391 |
| 40 |
9.5 |
39.7 |
0.5054 |
*Lambda (λ, taken from the Poisson Distribution formula) is equivalent to the plating density (number of viable cells/well) ƛ = -ln(growth negative wells/total wells) = average plating density (cells/well)
To assess the 25 μL and 40 μL fill 384-well agar plates, dragonfly discovery was employed to inoculate the agar-filled plates with individual cells of L. crispatus from a culture diluted to extinction.
The stock culture was diluted in MRS broth to a density of 2 x103 CFU/mL to achieve an average density of 0.5 bacteria/well.
dragonfly discovery was subsequently employed to dispense 500 nL of culture to 368 wells of every plate, leaving column 24 uninoculated. Plates were then incubated anaerobically for 72 hours at 37 ̊C. Wells exhibiting colony growth were counted manually.

Image Credit: SPT Labtech
Results
With a plating density of 0.5 bacteria/well, the anticipated proportion of wells exhibiting growth was 39.3 %, as per Poisson distribution. For the 40 μL and 25 μL fill plates, the proportion of positive wells was 39.7 % and 25.3 %, respectively (as shown in Table 1).
Within the wells, colony growth manifested across the entire agar surface area, extending along the corners and side walls (shown in Figure 1).
Numerous attempts were made to enumerate positive wells by optical density reads of the plates, including well scans. However, this yielded variable results and was deemed unsuitable for data collection. This was likely the result of the off-center position of the colonies and the fluctuation in the depth of agar away from the well center due to the meniscus.
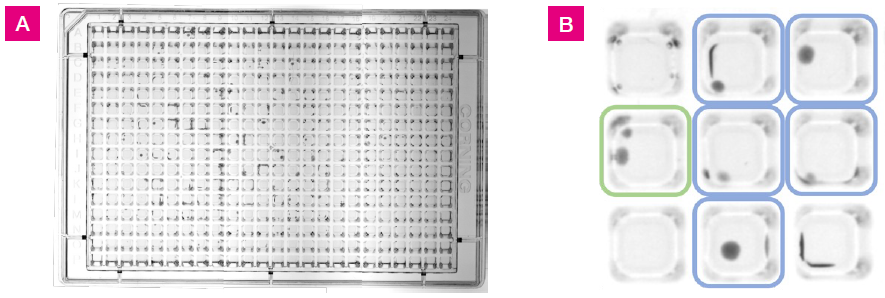
Figure 1. L. crispatus colony growth at 48 hrs on 384-well agar plate with 25 μL fill volume. A) Images of the plate were taken using a Syngene GBOX imaging system. This image was generated by stitching together three separate images. B) A section of the plate from wells G3 to I5. Colored squares indicate growth positive wells with one colony (blue) or two colonies (green). Colonies were located in the well centers and along the well walls. Image Credit: SPT Labtech
Conclusion
Given the anticipated proportion of growth-positive wells, this study concluded that the 40 μL fill volume was more suitable for culture application. The 25 μL fill volume yielded approximately 36 % less growth-positive wells than anticipated.
One hypothesis for this is that the trajectory of the inoculum dispense droplet may be angled in a way that the droplet contacts the side wall of the well, instead of the agar. The likelihood of this decreases in a 40 μL fill plate, as the distance the droplet travels from the syringe is not as long.
About SPT Labtech
SPT Labtech designs and manufactures robust, reliable and easy-to-use solutions for life science. We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the heart of what we do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A problem-solving state of mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.