Dr. Olof Beck’s pioneering contributions to analytical toxicology have shaped a range of exciting innovations in exhaled breath sampling and blood micro-sampling technologies.
Innovations in alcohol biomarker and drugs of abuse testing
The field of alcohol and drug abuse testing is rapidly evolving, with new drugs continually emerging and new methods for their accurate detection and quantification continuing to be required.
Dr. Olof Beck, Professor Emeritus at the Department of Clinical Neuroscience at Karolinska Institute (KI), Stockholm, Sweden, has witnessed these changes firsthand throughout his extensive career.
He has worked and conducted his research at KI for more than four decades, contributing to one of the world’s foremost medical universities with the country’s widest range of education in health sciences and medicine.
Dr. Beck’s research interests are in method development in pharmacology and toxicology, most notably mass spectrometry (MS), alcohol biomarkers, and toxicology.
His most recent work looks into breath testing for abused drugs, new psychoactive substances, dried blood spots, and the alcohol biomarker phosphatidylethanol.
Dr. Beck is a highly regarded scientist in bioanalysis and a pioneer in alternative sampling technologies. He has also founded or consulted for several start-up companies focused on innovations in patient sampling approaches.

Dr. Beck has worked with Waters for two decades and now uses the Waters Xevo TQ-XS System to support exhaled breath testing. Image Credit: Waters Corporation - Forensics
Working with Waters
Dr. Beck began working with ultra-high performance (UPLC-MS/MS) instrumentation from Waters around two decades ago, as the drugs of abuse testing field began moving away from GC-MS techniques and towards LC-MS techniques.
He eventually began working with the Waters Xevo™ TQ-XS system as part of his exhaled breath work and more recent research efforts. Dr. Beck cites support from Waters as one of his most valuable assets over the years:
“The support team for Waters in Sweden is very good. Mass spectrometers are complicated instruments, and you always need service and support. The Waters service personnel are very experienced and skilled. That’s important in a clinical laboratory setting because your instruments need to be running every day.”
A notable innovation is the Breath Explor, owned and manufactured by Munkplast AB based in Uppsala, Sweden. This instrument can measure drug use in almost any setting using exhaled breath.
Dr. Beck founded Capitainer® with several colleagues in 2016, aiming to develop the Capitainer®qDBS-quantitative microfluidic dried blood sample devices suitable for home use.
More recently, he has begun consulting for ABC Labs, a private company working to develop digital infrastructure around toxicology testing that can better integrate healthcare providers, patients, and laboratories to make diagnostics more accessible, affordable, and readily available.
These innovations were all made possible thanks to the technical evolution of liquid chromatography coupled with tandem MS (LC-MS/MS) technology. These advances have helped address many of the challenges associated with the conventional immunoassay methods used in toxicology.
Dr. Beck outlines how LC-MS/MS has contributed to development in the field, transforming analytical methods for drugs of abuse and alcohol testing:
“In this field, the number of analytes that we need to detect is always increasing. New psychoactive substances and therapeutic drugs constantly need to be added to testing panels.
“With an increasing number of analytes, you would have to run many immunoassays for each sample, so it becomes costly. Then, with the development of oral fluid testing, the reagents for immunoassay screening in oral fluids had cutoff limits that were too high, and they were not stable for the application.
“At the same time, the performance of LC-MS/MS instruments has improved significantly, making it a better choice for testing laboratories. Today, you cannot detect these new drugs with immunoassays, you must use LC-MS/MS.”

Dr. Beck is a founding member of Capitainer and also recently began consulting for ABC Labs, a private company developing a digital infrastructure around toxicology testing. Image Credit: Waters Corporation - Forensics
Dr. Beck has relied on the Waters Xevo™ TQ-XS Tandem Quadrupole Mass Spectrometer in combination with the ACQUITY™ I Class UPLC™ system in all these endeavors, as well as his own academic research.
These tools provide a robust and suitable system able to offer the sensitivity required for sample-limited toxicology applications.
Drugs of abuse and alcohol testing
Drug testing via urine samples has been the standard in clinical and forensic applications since the 1970s. This followed the development of immunochemistry-based analytical technologies that enabled the cost-effective screening of common drugs of abuse via gas chromatography-mass spectrometry (GC-MS) methods to achieve the evidential confirmation of positive outcomes.
Developments in MS technology since that time mean that it is now possible to avoid immunoassay screening entirely, instead using evidential LC-MS/MS methods to directly make an analytical investigation. This shift has changed the paradigm.1
Interest in alternative matrices arose in the early days of drug testing due to the limitations and potential for fraud associated with reliance on urine samples. Alternative sampling technologies are becoming increasingly common in routine applications, a development that stems from the development of more powerful bioanalysis-based LC-MS/MS instruments that have set a new standard of method performance.2 Dr. Beck has seen these developments firsthand:
“I was a GC-MS person from the early 1970s, and I was really biased toward this technique. However, when we started using LC-MS/MS, I saw the light. In the early 2000s, when UPLC came about, we saw its potential.
“We started with Waters Quattro Premier instruments, which solved several problems associated with using GC-MS for confirmation analysis, including laborious sample preparation, long run times and a complicated and lengthy validation processes.
“Customers, of course, were frustrated with these very long reporting times. We started with a concept called dilute and shoot, which I helped to pioneer. Using the separation power of UPLC, we could replace all the complicated GC-MS methods with LC-MS/MS direct injection. It was very successful.”
Dr. Beck added the Waters TQ-XS Tandem Quadrupole Mass Spectrometer to his laboratory setup, which has since played a major role in his many years of patient sampling innovations.
He has also taken advantage of numerous opportunities to collaborate with other leaders in alternative sampling, including Dr. Michael Böttcher, Head of Drugs and Drug Analysis at MVZ Medizinische Labore Dessau Kassel GmbH.
Dr. Böttcher is also at the forefront of the development and optimization of pioneering and increasingly popular oral fluid testing methods, as well as capillary blood testing. The developments are enabled by the Waters Xevo™ TQ-XS Mass Spectrometer. Dr. Beck outlines the impact of this instrument on his work:
“I started to use the Waters Xevo TQ-XS instruments for my work on exhaled breath specimens. That’s a really demanding application that requires very high sensitivity. Since then, I’ve continued to rely on the Waters Xevo TQ-XS, recently installing them in a new laboratory that I’m helping to start.”
Finger-prick blood sampling
Using a combination of paper and polymer microfluidics, Dr. Beck and his colleagues have developed a new blood sampling device called the Capitainer®qDBS (currently known as the Capitainer®B) device.
This device is now owned and distributed by Capitainer of Solna, Sweden. It allows anyone to obtain a precise 10 µL volume of blood from a finger prick and empowers patients to acquire a blood sample for clinical analysis at any time and in any place without adversely affecting testing accuracy.
The technology allows the immediate application of blood from the finger prick to a pre-prepared DBS filter paper to facilitate drying.
This workflow affords the user considerable time savings, reducing or even removing the need for downstream sample preparation since the circular discs punched from the filter paper can function as the sample for an array of downstream testing applications.
DBS testing is typically employed alongside sensitive analytical technology such as mass spectrometry, meaning that blood volumes in the microliter range are sufficient. Dr. Beck summarizes the technology’s origins:
“I was doing malaria research in Uganda with one of my PhD students, and they were trying to take venipuncture samples in the field, then using a pipette to store it on filter paper and bring it to the lab.
“That was 17th-century technology, so I thought we needed something better. We figured it out, published our findings in 2015, applied for a patent, and then started Capitainer.”

Capitainer has developed quantitative microfluidic dried blood sample devices for home use, known as the Capitainer®qDBS. Image Credit: Waters Corporation - Forensics
The device’s high volumetric accuracy and precision mean that sample collection is equal to industrial pipetting. It is also as reliable as venipuncture, which is routinely employed throughout the healthcare system.
It is possible to acquire samples anywhere without the need for trained personnel to be present. This allows self-sampling and at-home or remote testing, significantly expanding testing possibilities in regions with limited access to medical professionals.
These dried samples can survive for up to one year at room temperature, meaning that they can then be sent for a range of laboratory analyses.
Dried blood samples’ stability also means that this technology is ideally suited to testing in developing countries where it is necessary to send samples to a laboratory far away, potentially even in another country. This method can also be less intrusive and more resistant to fraud than urine sampling, as Dr. Beck highlights:
“From a finger prick, we can do alcohol biomarkers and drugs of abuse screening, as well as measure the exact concentration of methadone in patients who are on methadone treatment.
“The clinicians were very accepting of the idea to get everything in just one finger prick. If you do finger prick sampling in the correct way, there is no pain. You just put a bandage over it, and the next day it’s healed. When the sample arrives at the laboratory, it’s exactly 10 µL.
“It’s more accurate than pipetting. Then you just use it for analysis directly. You just take the disc and put it into the system directly. It’s possible to automate it as well in a 96-well format for high-volume applications.”
Its quantitative properties and ease of use mean that Capitainer®qDBS can be used for home sampling in large populations. It is ideally suited to monitoring applications such as hormone levels, viral infections, or biomarkers such as NT-proBNP (cardiovascular diseases) or PSA (prostate cancer).
Immunocompromised individuals, such as transplant patients, could also greatly benefit from the technology. It has the potential to reliably monitor these patients from their own homes, meaning that they can better avoid pathogen exposure when going to the hospital for testing.
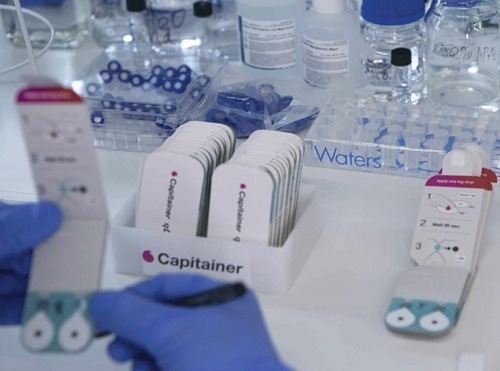
Capitainer®qDBS samples can be used for accurate quantitative analysis, which opens the technology to almost endless possibilities. Image Credit: Waters Corporation - Forensics
Dr. Beck has consulted on studies looking into other ways that Capitainer®qDBS samples can be employed in accurate quantitative analysis, opening the door to potentially endless possibilities.
For example, SciLifeLab researcher Jochen Schwenk and his team published an article in 2021 showcasing how dried blood samples from the Capitainer®qDBS device could be as accurate as existing plasma sampling for monitoring SARS-CoV-2 infection.3
The study highlighted the technology’s potential, prompting much interest in collaborations. The Public Health Agency of Sweden (FHS) has used the qDBS technology in three large COVID-19 monitoring studies, and SciLifeLab plans to develop the device further to enable proteomic profiling and the potential quantification of other molecules.
Exhaled breath
Dr. Beck’s research on exhaled breath led to the development of the Breath Explor, a patient sampling device owned and manufactured by Munkplast AB in Uppsala, Sweden.
Other than from volatiles, exhaled breath also includes aerosol particles that carry non-volatile components from deeper parts of the lung.4 It is possible to analyze samples using MS in order to determine the presence of clinical biomarkers or drugs of abuse.
The device has been developed for professional use in clinical studies, workplace drug testing, dependence clinics, and the monitoring of therapeutic drugs. Dr. Beck outlines its evolution:
“The discovery I made back in 2010 is that you can collect aerosol particles during normal breathing. Every time you breathe, you also produce some microparticles from the deeper part of the lung as part of the breathing process.
“If your body is contaminated with the drug, you will see it in the particles. But you can also use these particles for other biomarkers or inflammation. For example, you can measure therapeutic drugs for asthma using MS to confirm that they are present in the right compartment of the lung.”
The Breath Explor sampling device is comprised of a collector unit with three separate collectors, a body, and two caps. The device can be disassembled with ease to facilitate both manual and automated robot sample preparation.
Its three collectors mean that the Breath Explor can accommodate multiple test options from the same sample, making it an attractive option for forensic, clinical, and research purposes, where there may be a need for diverse or confirmatory testing. Dr. Beck outlines how the device can be used in the field:
“Traditionally researchers might go out to nightclubs and ask people whether they are intoxicated or not, or whether they used cocaine recently. Now they can ask to take a sample too.
“We published a study5 where 4.3% of the interviewed persons self-reported that they’ve been using drugs recently. When we did the analysis, 13% were intoxicated. People don’t confess to it, which affects the accuracy of the research.”
A new approach to toxicology testing
Dr. Beck’s most recent endeavor involves consulting for ABC Labs, which was built and deployed over 7 weeks in response to the unprecedented challenges of the COVID-19 pandemic.
ABC closely collaborated with the Swedish Public Health Agency, eventually performing up to 25% of all national testing for Covid-19.
The lab created unique products via partnerships with academic scientists, enabling the Swedish national screening program for the Alpha, Delta, and Omicron variants of the virus, as well as the first test for measuring T cell immunity in Europe. Dr. Beck highlights his initial interest in the company:
“ABC Labs could provide 24/7 service for Covid-19 tests, and they analyzed about 30,000 samples each day with a 24-hour response time. I was really impressed how they developed operations of that size in a very short time. I want to do the same with toxicology.”
Dr. Beck is supporting ABC Labs in developing other services, including preventive health screening and toxicology testing. His consulting position involves establishing a toxicology laboratory for workplace and clinical testing that leverages LC-MS/MS to offer a 24-hour reporting time on multi-panels for abused drugs and alcohol biomarkers.
The laboratory will also employ some of Dr. Beck’s own innovations in its work, including devices for exhaled breath and dried blood spots. He explains the rationale:
“I got the opportunity to set up an analytical toxicology unit according to my own ideas and build something new that we can make profitable. In drugs of abuse testing, you usually do immunoassay screening and then you do confirmation by MS. My idea was to only use MS for screening and confirmation, and that’s what we are establishing in this new laboratory.
“We don’t do any immunoassay at all. We will also use LC-MS/MS for alcohol biomarkers. Right now, we have about 40 substances in our testing panel, and we’re setting up special panels for stimulants, opioids, synthetic cannabinoids, and more.
“We’re estimating we can run about 2,000 samples per day. When I began to set up the testing laboratory at ABC, I decided to only use the Waters Xevo TQ-XS instruments as standard. Even if the instrument is more expensive, it’s more productive.”

Breath Explor uses exhaled breath to measure drug use in almost any setting. Image Credit: Waters Corporation - Forensics
ABC Labs will fill a gap in workplace drug testing for employers across Sweden once the laboratory is up and running. Dr. Beck expands on the potential impact:
“We have a population of 10 million people in Sweden and carry out around 300,000 workplace drug testing samples per year. I’ve been working very closely with the dependency clinic here in Stockholm, so I know them very well. I’m running clinical studies together with them, and I understand what they are demanding regarding alternative matrices and drug panels.
“I’ve been also very active in the field of workplace drug testing in Sweden. The concept of workplace drug testing has been well accepted here in Sweden, including by the employee unions, because we feel we have a very safe system.”
ABC Labs will also leverage some of Dr. Beck’s patient sampling inventions, particularly in terms of the option of testing for different sample types. He continues:
“The idea is to offer all these different specimens as part of the service. When oral fluid came about 20 years ago, people were expecting oral fluid to replace urine, but it didn’t. It has created some new markets where urine is not possible to use. So, I think in the future we will use all of them.”
Next steps
While technically retired from academia, Dr. Beck envisions his consulting work will continue as new patient sampling products evolve from his research and are developed by companies. He also sees potential for these devices to be used to support patients in novel and evolving ways:
“You can use this technology to determine if a candidate is at risk of alcohol abuse, for example, and provide the support to help the person reduce his/her drinking. Or maybe they are waiting for liver transplantation, and they must stop drinking to qualify.
“The concept is not designed to kick them out, but to get them the help they need. In some cases, they may not even need a transplant once they’ve stopped. So, it can support the patient, not just punish.”
References and further reading
- Rosano, T.G., et al. (2016). Definitive Drug and Metabolite Screening in Urine by UPLC-MS-MS Using a Novel Calibration Technique. Journal of Analytical Toxicology, (online) 40(8), pp.628–638. https://doi.org/10.1093/jat/bkw050.
- Takyi-Williams, J., Liu, C.-F. and Tang, K. (2015). Ambient Ionization MS for Bioanalysis: Recent Developments and Challenges. Bioanalysis, 7(15), pp.1901–1923. https://doi.org/10.4155/bio.15.116.
- Niclas Roxhed, Bendes, A., et al. (2021). Multianalyte serology in home-sampled blood enables an unbiased assessment of the immune response against SARS-CoV-2. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-23893-4.
- Beck, O., Olin, A.-C. and Mirgorodskaya, E. (2016). Potential of Mass Spectrometry in Developing Clinical Laboratory Biomarkers of Nonvolatiles in Exhaled Breath. Clinical Chemistry, 62(1), pp.84–91. https://doi.org/10.1373/clinchem.2015.239285.
- Feltmann, K., et al. (2022). Feasibility of using breath sampling of non-volatiles to estimate the prevalence of illicit drug use among nightlife attendees. Scientific Reports, 12(1). https://doi.org/10.1038/s41598-022-24741-1.
Acknowledgments
Produced from materials originally authored by Waters Corporation.
About Waters Corporation - Forensics
Address the constantly evolving challenges faced by forensic and toxicology labs with Waters LC-MS systems and informatics, proven LC-MS solutions that deliver data you can trust in forensic labs.
You have a challenging job to do, whether that’s identifying seized drugs or new psychoactive substances, presenting reliable forensic data in court, monitoring illicit drug use, or analyzing prohibited substances in a sports doping investigation.
With Waters as your partner, you’re never alone in overcoming those challenges. Get the critical data you need with our powerful forensic solutions portfolio, including industry-leading liquid chromatography, mass spectrometry, informatics, chemistries, and comprehensive support. At Waters, we’re committed to advancing science in partnership with our customers, and we love to prove it.
For forensic toxicology use only.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.