Forensic toxicology laboratories rely on reliable screening techniques to detect a wide variety of toxicants in highly complex biological matrices, such as ante- and postmortem specimens.
In 2009, Waters released its targeted toxicology screening application using the ACQUITY™ TQD System.1 Rosano et al. leveraged this approach to compare screening methodologies for postmortem blood samples.2
This solution proved to be successful and was later transferred to the ACQUITY™ UPLC™ I-Class and Xevo™ TQD system in 2013.3 The subsequent release of the Xevo™ TQ-S micro has allowed this solution to evolve further.4

Figure 1. ACQUITY UPLC I-Class and Xevo TQ-S micro configuration. Image Credit: Waters Corporation - Forensics
By combining the ACQUITY™ UPLC™ I-Class with the Xevo™ TQ-S micro, this established UPLC-MS/MS screening methodology can now be used with the latest generation of Waters' mass spectrometers.
Experimental
Test substances
Several commercially available human urine reference controls were obtained:
- Basis-line U from Medidrug (40201) (ACQ Science)
- Blankcheck urine (UR015) and DCT -25% (UR22020A) (ACQ Science)
- Urine Toxicology Control DAU HC2 (50701) (UTAK)
- Liquichek Urine Toxicology Quality Controls (Bio-Rad): Negative Control (460), C2 (442), and S10 (673)
Sample preparation
These reference urines were diluted 5-fold with mobile phase A before vortex mixing. The supernatant was transferred to a Waters Maximum Recovery vial after centrifugation, with analysis performed on triplicate injections.
LC conditions
Source: Waters Corporation - Forensics
| |
|
| System: |
ACQUITY UPLC I-Class with FTN |
| Column: |
ACQUITY UPLC HSS C18, 100A,
1.8 μm, 2.1 mm x 150 mm (P/N 186003534) |
| Column temp.: |
50 °C |
| Sample temp.: |
10 °C |
| Injection volume: |
5 μL |
| Wash solvent: |
Acetonitrile/water (95:5 v/v) |
| Purge solvent: |
5 mM ammonium formate pH3.0 |
| Flow rate: |
0.4 mL/min |
| Mobile phase A: |
5 mM ammonium formate pH3.0 |
| Mobile phase B: |
Acetonitrile containing 0.1% formic acid |
MS conditions
Source: Waters Corporation - Forensics
| |
|
| System: |
Xevo TQ-S micro |
| Ionization mode: |
ESI+ |
| Capillary voltage: |
3.0 KV |
| Source temp.: |
150 °C |
| Desolvation temp.: |
400 °C |
| Desolvation gas: |
800 L/Hr |
| Cone gas: |
20 L/Hr |
| Cone voltages: |
Preconfigured in provided MRM method |
| Collision energies: |
Preconfigured in provided MRM method |
Results and discussion
Data was collected using the supplied MRM method. This method includes two transitions (qualifier and quantifier) per compound, with associated preconfigured parameters for cone voltage, as well as collision energies for a total of 178 compounds.
Three negative control reference urines (Basis-line U, Blankcheck, and Negative Control) and four positive control reference urines (C2, S10, DAU HC2, and DCT -25%) each contained certified levels of analytes.
These were assayed using the method described here, with data automatically processed via the TargetLynx Application Manager. The area threshold reject parameter was slightly increased, a result of the TQ-S micro’s increased response.
Screening results were evaluated to assess equivalence between results and the data obtained from the Xevo™ TQD platform.
Several compounds, including caffeine and other substances linked to over-the-counter medications, were detected in the negative control reference urines on both platforms. These compounds are regularly detected in urine screens.
In the case of the certified positive control reference urines, both platforms detected an identical number of expected compounds in the S10 reference urine.
The Xevo™ TQ-S micro and the Xevo TQD each detected the same analytes in the C2 and DAU HC2 urine samples, but the Xevo™ TQ-S micro was also able to detect α-hydroxyalprazolam in the C2 urine and lorazepam in the DAU HC2 urine.
Further sensitivity to benzodiazepines was confirmed via analysis of the ACQ Science DCT - 25% sample.
For example, Figure 2 features five additional benzodiazepines detected using the Xevo™ TQ-S micro. This commercial reference urine features certified analyte levels at a concentration equivalent to 25% less than the maximum cut-off concentration currently advised by the European Workplace Drug Testing Society (EWDTS) for confirmation tests in urine.5
In the example presented here, detected benzodiazepines are present in this urine at 75 ng/mL.
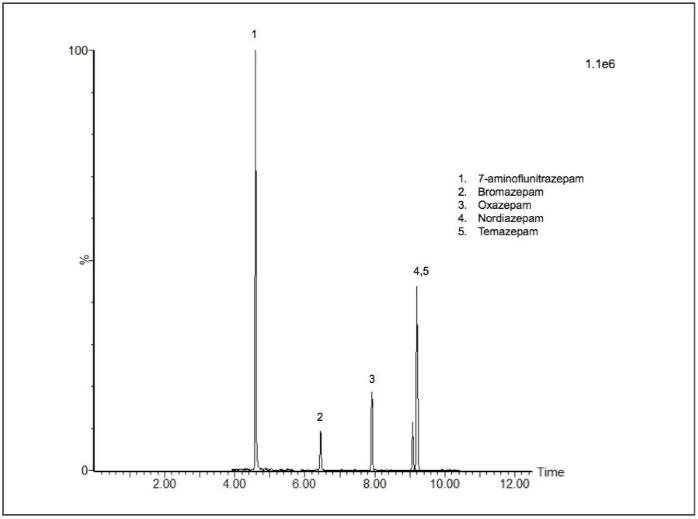
Figure 2. Chromatogram showing benzodiazepines in the ACQ DCT -25% commercial reference urine detected by the Xevo TQ-S micro using the supplied targeted MRM method but not the Xevo TQD. The quantifier ion transition is displayed. Image Credit: Waters Corporation - Forensics
It was observed that the average number of scans per function has increased as the method has evolved from the ACQUITY™ TQD System through the ACQUITY™ UPLC™ I-Class/Xevo TQD and onto the ACQUITY™ UPLC™ I-Class/Xevo™ TQ-S micro.
The dwell time (10 msec) in the supplied MRM method remains the same, however, meaning that this increase can be attributed to improvements in electronic design found in each new MS platform. This increased number of scans per function is key to enhancing reproducibility, precision, and sensitivity.
References and further reading
- Waters (2025). Targeted MRM Screening for Toxicants in Biological Samples By UPLC-MS/MS. (online) Available at: https://www.waters.com/nextgen/be/en/library/application-notes/2008/targeted-mrm-screening-for-toxicants-in-biological-samples-by-uplc-ms-ms.html?srsltid=AfmBOor4obzQ8qUeIf1jhtPlpa2HDX3BA9N1OkSqPXP_QBzCxitq2L9- (Accessed 23 Apr. 2025).
- Rosano, T.G., Wood, M. and Swift, T.A. (2011). Postmortem drug screening by non-targeted and targeted ultra-performance liquid chromatography-mass spectrometry technology. Journal of analytical toxicology, (online) 35(7), pp.411–23. https://doi.org/10.1093/anatox/35.7.411.
- Waters (2019). Forensic Toxicology Screening Using the ACQUITY UPLC I-Class System with the Xevo TQD. (online) Available at: https://www.waters.com/nextgen/in/en/library/application-notes/2013/forensic-toxicology-screening-using-acquity-uplc-i-class-system-xevo-tqd.html?srsltid=AfmBOoqzBVbOKi6V4acbIzKUPhqfUDJyiA7pX12cEV80wfc2HXxpNOcJ (Accessed 23 Apr. 2025).
- Xevo TQ-S micro Product Brochure. 2024.02 Waters Marketing Brochure, 720005046EN. Available at: https://www.waters.com/nextgen/us/en/library/library-details.html?documentid=720005046&t=waters-XevoTQSmicroProductBrochure-720005046
- European Workplace Drug Testing Society. Available at: https://www.ewdts.org/wp-content/uploads/2022-10-ewdts-guidelines-urine-final.pdf.
Acknowledgments
Produced from materials originally authored by Robert Lee and Michelle Wood from Waters Corporation.
About Waters Corporation - Forensics
Address the constantly evolving challenges faced by forensic and toxicology labs with Waters LC-MS systems and informatics, proven LC-MS solutions that deliver data you can trust in forensic labs.
You have a challenging job to do, whether that’s identifying seized drugs or new psychoactive substances, presenting reliable forensic data in court, monitoring illicit drug use, or analyzing prohibited substances in a sports doping investigation.
With Waters as your partner, you’re never alone in overcoming those challenges. Get the critical data you need with our powerful forensic solutions portfolio, including industry-leading liquid chromatography, mass spectrometry, informatics, chemistries, and comprehensive support. At Waters, we’re committed to advancing science in partnership with our customers, and we love to prove it.
For forensic toxicology use only.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.