German company MVZ Medizinische Labore Dessau Kassel GmbH (MVZ) has positioned itself as an industry leader in the development of rapid, sensitive means of testing for drugs of abuse using capillary blood and oral fluid samples.
Drugs of abuse testing and monitoring at MVZ
MVZ prides itself on the company’s pioneering capabilities in drugs of abuse testing and monitoring, particularly in applications involving intoxication, drug screening for clinical and forensic applications, drug level determination, and subsequent confirmatory analysis.
The company’s laboratory is in Dessau, Germany, with the 35 employees in its Toxicology Department working 24/7 to analyze a range of samples for drugs of abuse, including opiates and opioids (including substitutes), analgesics, benzodiazepines, and synthetic cannabinoids.
The team also analyzes therapeutic drugs, including other sedatives, neuroleptics, psychoanaleptics, antidepressants, antiepileptics, and antiarrhythmics.
The team works with a variety of sample types that are analyzed in the laboratory, including urine, hair, capillary blood, and oral fluid.
The company’s clients include health clinics, prisons, psychiatric hospitals, and specialist psychiatric prisons, as well as private physician practices working in addiction medicine.
The laboratory offers a range of ISO 17025 and ISO 15189 accredited services, supporting its customers with the development of new psychoactive substances, monitoring illicit drug use, the presentation of dependable forensic data in court, or the analysis of prohibited substances as part of investigations.
The forensic and clinical toxicology testing laboratory was historically reliant on urine samples, but these samples tend to present major challenges during the collection process.
For example, sample collection must be supervised to avoid sample substitution or adulteration, which presents an increased risk compared to other types of biological samples.

MVZ Medizinische Labore Dessau Kassel GmbH offers cutting-edge capabilities in drugs of abuse and therapeutic drugs testing. Image Credit: Waters Corporation - Forensics
Working with Waters
MVZ began working with Waters when the laboratory started to invest in LC-MS/MS instrumentation to improve its throughput. The two companies have worked closely together since 2007, as the MVZ team continued to develop the innovative methodologies necessary for the detection of new drug compounds.
MVZ’s Head of Toxicology, Dr. Michael Böttcher, and his team have especially benefited from the support received from Waters over their years of working together. He outlines this appreciation:
“Waters technical engineers can service both their chromatography and MS instrumentation, so we’re working with the same people for each. That’s very different from our experience with other vendors.
“When you use equipment from multiple manufacturers, problems can cause a blame game, with one company suggesting the issue is with the other’s instrument.
“There’s a tendency to avoid taking responsibility for mistakes in either their instrumentation or their support. With Waters, it’s the same company, so they will work to solve the problem, no matter what.”

Dr. Michael Böttcher, MVZ’s Head of Toxicology, and his team have appreciated the support they’ve received from Waters since they began working together in 2007. Image Credit: Waters Corporation - Forensics
Working under Dr. Böttcher’s direction, the company has successfully developed and optimized a range of pioneering capillary blood testing and oral fluid testing methods, the latter of which is gaining increased popularity.
Samples can be collected using either a thumb prick or swab. These groundbreaking tests can be supervised more easily, reducing the opportunity for fraud. Dr. Böttcher continues:
“We understood the challenges in traditional urine sample collection and analysis, and we saw an opportunity to develop new testing solutions that address these industry-wide problems.
“We’ve worked to develop highly sophisticated methods for oral fluid samples, something that has established us as a leader in the market. We currently receive and test approximately 600-700 oral fluid samples per day, which are analyzed using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS).”
MVZ has invested in a range of analytical instrumentation and software from Waters, working closely with Waters’ technical support team to develop and optimize the company’s industry-leading drugs of abuse testing services for capillary blood and oral fluid samples.
LC-MS in forensic and clinical toxicology
Like many laboratories, MVZ historically employed gas chromatography coupled with mass spectrometry (GC-MS) during the company’s early years. This approach was widely considered to be the industry’s gold standard.
Since then, LC-MS methods have become more popular as scientists continue to search for improvements in workflows, most notably in terms of reduced sample preparation, increased sample throughput, and ease of use. These improvements must be balanced with maintaining the high sensitivity required for these applications.
Those capabilities prompted MVZ’s initial investments in highly sensitive LC-MS instruments. These instruments enabled the more rapid identification and quantification of parent drugs and their corresponding metabolites, providing valuable analytical data for their customers. Dr. Böttcher explains:
“Originally, we were interested in LC-MS because of its capability to improve our laboratory throughput, giving us better workflows with the sensitivity we needed.
“LC-MS offered significant advantages for sample preparation, making it much more efficient and cost-effective for us. That’s important in a time-sensitive industry like this one. Post-run time is also greatly reduced in LC-MS compared to GC-MS, and data analysis is more efficient.”
Drug tests in forensic laboratories traditionally involved a two-step process made up of an immunoassay screen followed by confirmatory analysis via GC-MS or LC-MS/MS.
This process can result in a high number of false positives when used in some applications, potentially missing drugs with negative results. For example, the majority of commercial opiate immunoassays are unable to detect fentanyl or fentanyl analogs.
LC-MS analysis saves both costs and time for companies like MVZ because it eliminates presumptive immunoassay analysis. It is also able to detect all significant drugs present in the sample, quantifying drug concentrations that reflect actual impairment.
This technique can be used to detect a diverse array of drugs and other substances at very low concentrations, an important consideration in toxicology testing where even trace amounts of a substance can be significant.
LC-MS is a valuable tool for a multi-targeted screening approach because it can be used to accurately identify and quantify multiple substances in one sample. LC-MS can also facilitate a quantitative follow-up on drugs with long detection-time windows, including THC and benzodiazepines.
LC-MS is particularly beneficial to MVZ’s forensic toxicology customers because it can be used to acquire detailed information about a substance’s chemical structure, which can be used to confirm the identity of a compound and distinguish this from other substances potentially present in the sample.1
This approach is also relatively fast and able to process significant numbers of samples in a short time period. These characteristics make LC-MS highly suited for use in high-throughput toxicology testing. Dr. Böttcher explains:
“If our clients need tests that will be used in court, for example, we’re able to provide them with results from an accredited lab that can prove a specific substance has been detected. We also provide quality control measures to verify the results.”
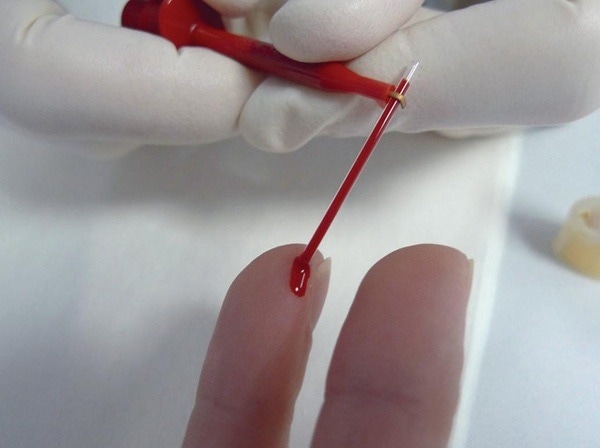
Capillary blood testing offers a new route for drug testing, as it is more easily supervised and reduces the opportunity for fraud. Image Credit: Waters Corporation - Forensics
Pioneering testing methodology
MVZ’s extensive hands-on experience in forensic and clinical toxicology affords the company a distinct perspective on the industry's challenges, especially the inherent disadvantages of the urine sample collection process.
The laboratory developed innovative LC-MS methods to optimize oral fluids testing. These developments allowed the laboratory to expand its services while simultaneously attracting new customers. Dr. Böttcher outlines this process:
“Urine is the most common physiological fluid used for testing, but the samples must be collected under the supervision of a nurse or physician. That puts patients in an uncomfortable situation.
“Also, it can be challenging to develop new methods for urine testing that can detect new metabolites as drugs are introduced. About 10 years ago we began to research and develop new methods specifically for oral fluids using LC-MS.
“Now our customers are increasingly interested in testing services for other body fluids, particularly capillary blood and oral fluid.”
Saliva sample types can be collected quickly and easily, and they are non-invasive. This makes the testing process much more convenient for both the healthcare provider and the patient themselves.2
Oral fluid is potentially less susceptible to adulteration or tampering, a common concern with conventional specimen types such as blood or urine. Studies have also suggested that oral fluid and other sample types could offer enhanced specificity and sensitivity for certain drugs of abuse, making them more useful in the detection and monitoring of potential substance use.3
MVZ’s expanded service offerings prompted a need to maintain the more rapid workflows afforded by LC-MS. The company looked to technological advances in LC-MS instrumentation in order to achieve the improved sensitivity required for these types of analyses. Dr. Böttcher explains:
“We quickly learned that we needed better sensitivity for oral fluid samples. However, we also found that improved sensitivity could help with our methods for capillary blood samples because the sample sizes were smaller and detection limits were much lower.
“So, we decided to invest in very sensitive instruments from Waters to keep our sample preparation as simple as possible and to provide our customers with the time-sensitive results they need.”
Waters instrumentation and software
MVZ’s Toxicology Department leverages the powerful Waters ACQUITY™ UPLC™ I Class system in conjunction with the Waters Xevo™ TQ-XS mass spectrometer and MassLynx software in order to enhance the detection and quantification of the diverse array of drugs and metabolites routinely encountered in its drug testing work.
For example, the system’s wide dynamic range facilitates the confident detection of extremely potent fentanyl compounds at trace levels, as well as more common drugs present in higher concentrations.
The system’s improved selectivity, speed of analysis, and optimum analytical sensitivity mean that MVZ has the tools required to efficiently achieve accurate results. Remarking on these improvements, Dr. Böttcher says:
“Of course, the capabilities of LC-MS instrumentation have continued to improve through the years.
“As the sensitivity of the Waters instrumentation increased, we were able to establish the idea of what we call multi-target screening. Over time, we’ve expanded this service to include more drug compounds.
“Today, our multi-target screening in oral fluids can detect up to 73 substances from different classes. In parallel, we did the same thing for capillary blood samples, and we can now detect up to 81 substances.”
MVZ has benefited from the ease of use, cost-effectiveness, and increased sensitivity afforded by using the Waters UniSpray Ion Source (USI) on the Waters Xevo™ TQ-XS Mass Spectrometer. The USI’s unique geometry utilizes a number of different mechanisms to produce smaller droplets and improve desolvation.
These effects combine to generate more ions from the same amount of sample versus traditional ionization modes like electrospray, resulting in an increased response across a broad range of compounds.
The combination of a potential boost in response and a wider range of analytes means that several analysis methods can be consolidated into one, or the need to change over sources between analyses can be removed altogether. Both these capabilities help to save time and improve laboratory efficiency.
USI also allows the team to employ a post-column hydrochloric acid infusion, which is especially helpful for the sensitivity needed to perform capillary whole blood analysis.
For example, glucuronides (conjugates of glucuronic acid formed to improve solubility and kidney excretion), as combinations with often-toxic aromatic hydroxyl compounds, are extremely responsive to this combination.
The enhanced sensitivity is also beneficial given the short elimination times and half-lives of some compounds, and with substances like benzodiazepines, fentanyls, and buprenorphine that could be present at very low concentrations.
These capabilities have allowed MVZ to expand its range of services, most notably for capillary blood samples. Dr. Böttcher explains:
“With the addition of the Waters USI to the Waters TQ-XS Mass Spectrometer, we can test from a dried blood spot. We have cutoffs at 0.1 to 1.0 ng/mL. It’s been a game changer.
“Now we can offer capillary blood testing from a simple finger prick, which requires a sample volume of only 20 µL. That makes it very easy to take samples, so anyone can do it, and medical professionals are not required to be present in many settings.
“Plus, with such a small volume, our sample prep time is much faster, and we use less volume of solvents. So, it’s also more environmentally friendly and cost-effective.”
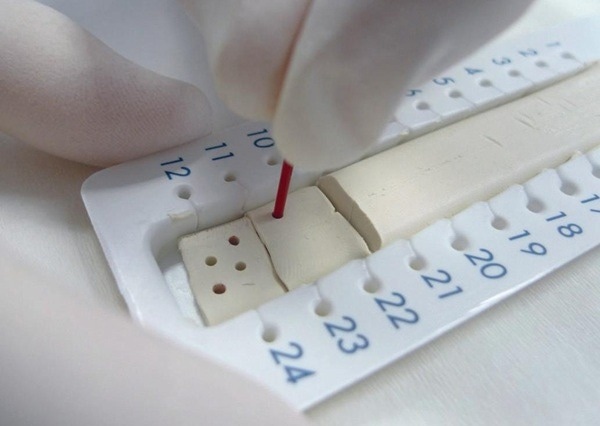
MVZ’s multi-target screening can now detect up to 81 substances in capillary blood samples. Image Credit: Waters Corporation - Forensics
Next steps
Ongoing and rapid changes in forensic and clinical toxicology mean that Dr. Böttcher and his team must continue to develop methods able to accommodate new substances, ensuring that the company can continue to provide faster and more sensitive drug screening.
The team depends on its access to the most current instrumentation and software to maintain this pace, and MVZ is considering further investments in new Waters instrumentation to accommodate specialized applications as it continues to expand its services and offerings.
Dr. Böttcher believes that ongoing advances in software are one of the most important technological developments in the field of routine drugs of abuse and forensic testing.
He predicts that developments in machine learning algorithms could enable more accurate and efficient analysis of test results, while improvements in user interfaces could result in easier-to-use and more intuitive software tools that offer laboratory technicians streamlined workflows while making it easier to achieve regulatory compliance.
Analyzing datasets rapidly and accurately could also help speed up the interpretation of laboratory results.
For example, in cases where each analyte and its associated deuterated standard are measured, it may be possible to simplify both laboratory processes and reporting to MVZ clients via an automatic pairing and presentation of a combined set of results:
“We see the advances in software as the key to further innovations in drugs of abuse testing.
“We’re watching closely as Waters continues to improve its software and will evaluate how these changes can help us further serve our customers and expand our capabilities. We want to build on our pioneering methodology and continue to generate new ideas and solutions in the future.”
References and further reading
- Neumann, J., et al. (2021). Ethyl glucuronide and ethanol concentrations in femoral blood, urine and vitreous humor from 117 autopsy cases. Forensic Science International, 318, p.110567. https://doi.org/10.1016/j.forsciint.2020.110567.
- Böttcher, M., Kühne, D. and Beck, O. (2019). Compliance testing of patients in ADHD treatment with lisdexamphetamine (Elvanse®) using oral fluid as specimen. Clinical Mass Spectrometry, 14, pp.99–105. https://doi.org/10.1016/j.clinms.2019.04.002.
- Böttcher, M., et al. (2019). Detection of heroin intake in patients in substitution treatment using oral fluid as specimen for drug testing. Drug and Alcohol Dependence, 198, pp.136–139. https://doi.org/10.1016/j.drugalcdep.2019.02.011.
Acknowledgments
Produced from materials originally authored by Waters Corporation.
About Waters Corporation - Forensics
Address the constantly evolving challenges faced by forensic and toxicology labs with Waters LC-MS systems and informatics, proven LC-MS solutions that deliver data you can trust in forensic labs.
You have a challenging job to do, whether that’s identifying seized drugs or new psychoactive substances, presenting reliable forensic data in court, monitoring illicit drug use, or analyzing prohibited substances in a sports doping investigation.
With Waters as your partner, you’re never alone in overcoming those challenges. Get the critical data you need with our powerful forensic solutions portfolio, including industry-leading liquid chromatography, mass spectrometry, informatics, chemistries, and comprehensive support. At Waters, we’re committed to advancing science in partnership with our customers, and we love to prove it.
For forensic toxicology use only.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.