The EU continues to publish new legislation on various topics with the potential to affect medical devices and IVDs. These new laws often include long and complex transition periods covering several years. This article will go over some of the new and evolving legislation that manufacturers must understand to ensure future compliance.
EU product safety legislation
To set the context, it is important to consider the underlying EU’s product safety legislation, which consists of three components:
- General Product Safety Regulation (GPSR) – Regulation (EU) 2023/988
- EU harmonization legislation (sectorial)
- Standards
The GPSR outlines the general safety framework for non-food consumer products in the EU. It functions as a safety net, encompassing products, components, and risks not addressed by harmonization legislation. It also ensures that products placed on the EU market are safe for consumers.
Sector-specific EU harmonization legislation summarizes the features and safety requirements necessary for specific products. Products placed in the EU market, such as medical devices, IVDs, personal protective equipment, and toys must comply with this legislation.
Standards are technical specifications implemented by a recognized standardization body, and compliance is not mandatory. There are four distinct categories of standards:1
- International – adopted by an international standardization body e.g., ISO
- European – adopted by a European standardization organization e.g., CEN or CENELEC
- Harmonized – adopted on the basis of a request from the Commission for the application of Union harmonization legislation2
- National – adopted by a national standardization body e.g. AFNOR
What is horizontal legislation?
Horizontal legislation is separate from product safety legislation and applies across multiple industries or policy areas. It sets out general principles or rules that span a variety of domains (e.g. competition law, environmental protection, consumer rights). For example, the General Data Protection Regulation (GDPR) is horizontal because it applies to all industries that handle personal data.
Horizontal legislation often provides the overarching principles (e.g. non-discrimination, consumer protection), while harmonization legislation applies these principles in certain industries. Harmonization laws must comply with horizontal legislation. For example, a harmonization product safety law must still respect horizontal consumer rights laws.
New and emerging legislation
Manufacturers sometimes overlook non-medical device and IVD regulations under the mistaken belief that these requirements are not needed for CE marking or will be addressed later by someone else in their organization outside of regulatory affairs. However, a single declaration of conformity must be drawn up, encompassing all applicable legislation at the time of CE marking.
As a result, a product can only be considered truly compliant when all relevant harmonization legislation and horizontal legislation has been addressed. Medical device notified bodies expect manufacturers to comply with applicable EU horizontal and harmonization legislation, especially when it intersects with the MDR or IVDR.
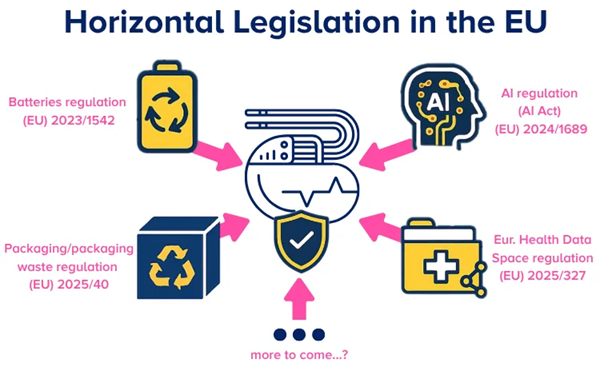
Figure 1. Horizontal legislation in the EU. Image Credit: RQM+
Recent regulations with potential impact on medical device and IVD manufacturers include:
- Batteries regulation (EU) 2023/1542
- AI regulation (AI Act) (EU) 2024/1689
- Packaging/packaging waste regulation (EU) 2025/40
- European Health Data Space regulation (EU) 2025/327
The following sections will go over each of these regulations in greater detail.
Batteries regulation (EU) 2023/1542
The batteries regulation is a new horizontal regulation published in the OJEU in July 2023. Some of its provisions began to apply on February 18, 2024, with the remainder introduced progressively over a period of more than five years. Requirements related to conformity assessment, CE marking, and the Declaration of Conformity have applied since August 18, 2024. The legacy batteries directive 2006/66/EC will be repealed on August 18, 2025.
This regulation establishes a comprehensive legal framework for the entire life cycle of batteries placed on the EU market. It applies to all battery categories, including:
- Portable batteries
- Light means of transport (LMT) batteries
- Starting, lighting, and ignition (SLI) batteries
- Industrial batteries
- Electric vehicle (EV) batteries
All batteries must undergo a conformity assessment, with requirements varying based on battery type and capacity. Medical devices and IVDs often (although not exclusively) use or contain portable batteries. Manufacturers of portable batteries must:
- Meet collection targets for waste portable batteries
- Mark batteries with separate collection symbols
- Mark batteries with the appropriate chemical symbol when containing > 0.002 % Cd, or > 0.004 % Pb
- Fulfill extended producer responsibilities (e.g. registration and reporting)
- Satisfy general battery labelling requirements
- Label rechargeable portable batteries with information regarding capacity
- Label non-rechargeable batteries with information regarding the minimum average duration
- Mark batteries with a QR code
- Meet minimum values for electrochemical performance and durability
The regulation also introduces the concept of removability and replaceability as well as battery due diligence.
Batteries must be engineered so they can be removed from a device without causing damage to either the battery or the product. Removal must be achievable using commercially available tools. This requirement applies to portable batteries used in products such as electronics, medical devices, and construction tools.
After removal, the battery must be replaceable - either with the original model or with a compatible one. Device manufacturers are required to supply replacement batteries as spare parts for at least five years after the product was last placed on the market.
Battery due diligence refers to a set of mandatory obligations for large companies to ensure responsible sourcing of raw materials used in batteries, with respect for human rights, labor standards, and the environment. Companies must implement a due diligence policy according to global standards and have both the policy and its implementation systems independently validated by a notified body.
AI regulation (AI Act) (EU) 2024/1689
The AI regulation (EU) 2024/1689 was published in the OJEU in July 2024. Its Date of Application is August 2nd, 2026, with certain provisions of the regulation concerning unacceptable risk AI systems applying from February 2, 2025.
The EU AI Act sets out harmonized rules for AI systems placed on the market, put into service, or used within the EU. It also prohibits specific AI practices. As a horizontal regulation, it applies to AI systems regardless of whether they fall under other EU legislation or not.
Medical devices and IVDs that employ artificial intelligence to any extent may fall within the scope of the AI Act. The regulation establishes four tiers of risk classification for AI systems, with proportionate control measures and monitoring activities. These categories, along with examples for each and control types are all displayed in Figure 1.
Under the AI Act, medical devices and IVDs that integrate an AI system are considered high-risk if they are classified above Class I under the MDR or Class A under the IVDR. For high-risk AI systems, requirements relating to conformity assessment, CE marking, and Declaration of Conformity will generally apply from August 2, 2027.
The AI Act applies to:
- AI system providers, whether established/located within the Union or in a third country
- AI system deployers established or located within the EU
- AI system importers and distributors
- Authorized representatives of providers located outside the EU
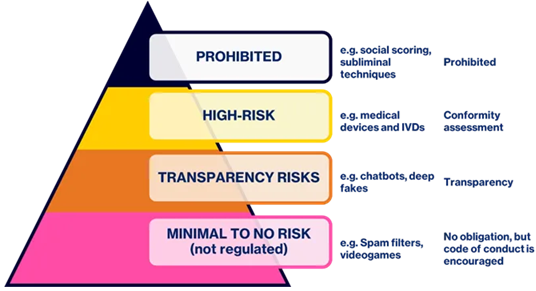
Figure 2. Categories of AI Systems.Image Credit: RQM+
Medical devices and IVDs that qualify as AI systems under the AI regulation and are placed on the market before August 2, 2027, are exempt from the regulation’s conformity assessment requirement. Any substantial alterations made to those devices after that date will necessitate a conformity assessment.
The regulation adopts the Notified Body model, which is already familiar to the medical device sector. It also introduces the European AI Board, composed of member state representatives, which serves as a coordination platform and advisory body to the Commission (similar to the role currently performed by the MDCG under the MDR/IVDR). The regulation additionally establishes a European AI Office responsible for supervising the general-purpose AI models.
The conformity assessment mandated by the AI regulation can be combined with the MDR/IVDR assessment and conducted by the same Notified Body if they are designated under the AI regulation. The AI regulation’s QMS requirements can be integrated into the QMS required by the MDR/IVDR. The Artificial Intelligence Board (AIB) and the Medical Device Coordination Group (MDCG) (AIB 2025-1 MDCG 2025-6) recently released guidance, offering valuable support in this area.
Packaging/packaging waste regulation (EU) 2025/40
The packaging/packaging waste regulation (EU) 2025/40 (abbreviated as PPWR) was published in the OJEU in January 2025. It is scheduled to apply from August 12, 2026, at which point the legacy directive 94/62/EC will be repealed. As a horizontal regulation, the PPWR covers all packaging and packaging waste generated from all sources such as commercial, household, and industrial settings.
The PPWR is based on the extended producer responsibility scheme and aligns with the EU’s New Circular Economy Action Plan (CEAP), which aims for all packaging to be economically reusable or recyclable by 2030. The PPWR applies to all medical devices and IVDs. However, due to the crucial nature of medical devices and IVDs, the obligations for producers are substantially reduced compared with those in other industries.
The regulation aims to ensure that packaging is designed, produced, and commercialized in a manner that enables high-quality recycling, maximizes reuse potential, and minimizes environmental impact during the full life cycle and the life cycle of the products for which it was designed.
Under the PPWR, packaging for medical devices and IVDs is defined as “contact-sensitive packaging”, which exempts medical devices and IVDs from most - but not all - PPWR requirements. The main requirements impacting medical devices and IVDs include:
- Packaging minimization
- Labelling
- Re-use targets
- Extended producer responsibilities
Manufacturers must perform a conformity assessment to verify that packaging they place on the EU market complies with the regulation’s sustainability, recyclability, and labelling criteria. The regulation does not mandate CE marking on packaging.
European Health Data Space regulation (EU) 2025/327
On March 25, 2025, the European Health Data Space (EHDS) regulation was published in the OJEU.
As harmonization legislation, it established a common framework for the use and exchange of electronic health data throughout the EU. Its objective is to support a single market for digital health services and products, promoting interoperability and innovation in electronic health record (EHR) systems.
The EHDS improves individuals’ ability to access and control their personal electronic health data and ensures that such data can be reused securely for research, innovation, policymaking, and regulatory purposes.
The regulation establishes primary and secondary uses of electronic health data.. Primary use supports healthcare delivery by allowing individuals and healthcare professionals to access, manage, and share personal electronic health data across borders within the EU. Secondary use refers to the reuse of health data for purposes beyond individual patient care, such as research, innovation, and policy-making.
The EHDS mandates that all electronic health record (EHR) systems comply with the specifications of the European electronic health record exchange format, ensuring EU-level interoperability.
In addition, the regulation will enable scientists and policymakers to access certain types of anonymized, secure health data, enabling the use of the EU’s extensive health data to support scientific study, develop improved treatments, and enhance patient care.
Under the EHDS, EHR systems are classified as products and must meet the essential requirements listed in Annex II. These requirements apply to the entire EHR system, rather than just individual software modules or harmonized components.
EHR system manufacturers are responsible for ensuring CE marking and compliance. The CE mark verifies that the EHR system satisfies EU requirements for safety, performance, and interoperability. This includes app and platform developers that allow patients or providers to access, store, or manage electronic health data in the priority categories (e.g. patient summaries, prescriptions, imaging, laboratory results).
Conclusion
The EU continues to introduce new legislation that impacts medical devices and IVDs. These laws often include extended transition periods, and producers must remain informed to ensure future compliance. When declaring conformity, manufacturers must assess all applicable legislation – not just the MDR/IVDR. This includes both horizontal and harmonization legislation. The next blog series will provide further insight into Medical Devices and Other EU Laws.
Related next steps
For difficulties in navigating the EU regulatory landscape or challenges in complying with horizontal EU regulations, RQM+ Corp’s experts can help manufacturers build comprehensive regulatory strategies designed to ensure that medical devices and IVDs comply with all applicable EU laws.
Acknowledgments
Produced from materials originally authored by Chris A. Parr, Principal, CRO at RQM+ Corp.
References and further reading:
- EUR-Lex. Regulation - 1025/2012 - EN - EUR-Lex. (online) Available at: https://eur-lex.europa.eu/eli/reg/2012/1025/oj/eng.
- EUR-Lex. EUR-Lex - union_legal_personality - EN - EUR-Lex. (online) Available at: https://eur-lex.europa.eu/EN/legal-content/glossary/legal-personality-of-the-eu.html.
About RQM+
Two industry leaders - R&Q and Maetrics - merged in 2020 to become RQM+, the largest medical device and diagnostics-focused full-service regulatory and quality consulting firm in the world. The integration of our leadership teams and the extensive skills and experience of our collective resources have created a highly agile and scalable global organization.
With offices in the United States, United Kingdom, and Switzerland, our large global footprint allows us to meet the regulatory and quality support needs of international clients of all sizes.
Order an Audit
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.