This article explores Europe, the EU, and the EU single market. The objective is to provide manufacturers with deeper insight into European countries and territories where CE marking enables market access.
Europe and the European Union (EU)
Before moving forward, the terms “Europe” and the “European Union” (EU) need to be defined. Europe is a broad geographical term referring to one of the world’s seven continents and all the countries located within it.
In contrast, the EU (see graphic below) is a political and economic union made up of 27 European countries that cooperate closely in areas such as trade, law, security, and environmental policy. Not all European countries are EU members; 22 European countries are not members of the Union.2
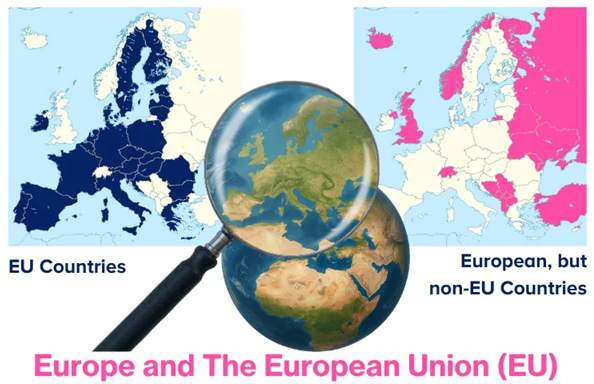
Image Credit: RQM+
The EU Single Market is a foundational element of the European Union, enabling the free movement of four essential elements throughout all EU member states. CE marking allows medical devices and IVDs to move freely within this market.
- Goods – Products can be traded across borders without tariffs or customs checks
- Services – Companies can provide services anywhere in the EU
- People – Citizens can reside, work, study, or retire anywhere in the EU
- Capital – Money can move freely for business, banking, or investment
European Free Trade Area (EFTA) and European Economic Area (EEA)
After defining the EU and its Single Market, the discussion now turns to the European Free Trade Association (EFTA) and the European Economic Area (EEA). EFTA is a regional trade organization and free trade zone consisting of four European countries. It was established as an alternative to the European Economic Community (EEC), the former name of the European Union.
EFTA facilitates free trade and economic cooperation among its members as well as with other countries around the world.
EFTA member countries:
- Iceland
- Liechtenstein
- Norway
- Switzerland
The European Economic Area (EEA) is a region that unites the 27 EU member states with three of the four EFTA countries (Iceland, Liechtenstein, and Norway), creating a single internal market. CE-marked medical devices and IVDs may move freely within the EEA.
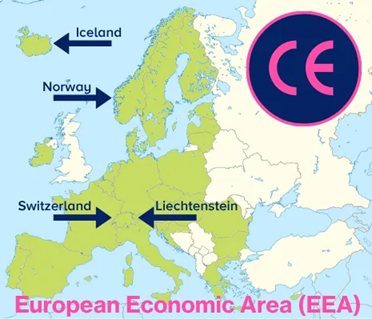
Image Credit: RQM+
EU Outermost Regions
The EU Outermost Regions (ORs) are territories of the EU member states France, Portugal, and Spain that, while located far from mainland Europe, are fully part of the European Union (see details and map below).
The name originates from Article 349 of the Treaty on the Functioning of the European Union (TFEU), which recognizes that these areas:
- Are geographically distant from continental Europe (often located in the Caribbean, Indian Ocean, or Atlantic).
- Deal with permanent constraints such as:
- Remoteness
- Insularity
- Small size
- Challenging topography and climate
Source: RQM+

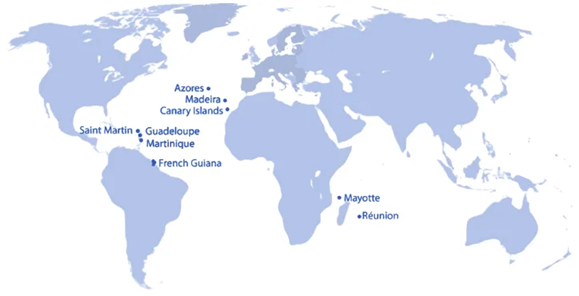
Map of European Outermost Regions.3 Image Credit: RQM+
These regions must follow EU law and benefit from special support due to their distinct challenges, including remoteness, insularity, and economic dependence. As a result, medical devices and IVDs marketed in the Outermost Regions must bear a valid CE mark, just as they would in mainland France, Spain, or Portugal.
Non-EU countries with Customs Union agreements
Turkey
Although Turkey is not an EU or EEA member, it is part of a Customs Union with the EU that includes medical devices and IVDs. As a result, medical devices and IVDs legally placed on the EU single market can also circulate in Turkey without extra customs duties or technical barriers, as long as they meet EU regulatory requirements. Devices must carry the CE mark to demonstrate conformity.
Andorra
Andorra, Monaco, and San Marino can all be described as European microstates. While independent from the European Union (EU), they each have special relationships with it.
Although Andorra is not a member of the EU or EEA, it has a customs agreement with the EU. Andorra lacks its own medical device or IVD regulatory framework, relying instead on EU regulations. This means that medical devices and IVDs placed on the Andorran market must be CE-marked. Devices must comply with MDR/IVDR for import or distribution within Andorra.
Monaco
Monaco is not a member of the EU or EEA, but it is in a customs union with France. Consequently, French and EU regulations apply, including those governing medical devices and IVDs. Medical devices placed on the Monaco market must carry a CE mark, as they must comply with MDR/IVDR through French oversight.
San Marino
San Marino is not a member of the EU or EEA, but it has close ties with Italy and a customs union with the EU. Medical devices and IVDs placed on the San Marino market must be CE-marked, as they must comply with MDR/IVDR through Italian oversight.
Switzerland
Switzerland had a Mutual Recognition Agreement (MRA) with the EU encompassing medical devices and IVDs. However, following the May 2021 implementation of the EU Medical Device Regulation (MDR), this agreement ceased to apply fully due to the lack of an updated institutional framework agreement between the EU and Switzerland.
Switzerland is not an EU or EEA member and now requires Swiss-specific regulatory compliance (MedDO).4 CE-marked medical devices and IVDs that meet MDR/IVDR requirements are unilaterally recognized by Switzerland.
United Kingdom
The United Kingdom of Great Britain and Northern Ireland formally exited the European Union on January 31, 2020. Great Britain (England, Scotland, and Wales) is no longer part of the EU and has no customs union agreement with the EU.
In Great Britain, manufacturers may apply the UKCA mark or the CE mark which is recognized until June 30, 2030 at the latest.5,6 Conversely, Northern Ireland remains in regulatory alignment with EU rules under the Windsor Framework Agreement.
Northern Ireland effectively remains part of the EU Single Market for goods only. As a result, medical devices and IVDs placed on the NI market require CE marking.
Summary
Medical device producers must understand the differences between Europe, the EU, the EEA, EFTA, and the EU Outermost Regions. These geopolitical and regulatory groupings decide market access, regulatory conformity, and requirements for product certification. In the EU and the EEA, medical devices and IVDs must comply with the MDR (EU) 2017/745 or IVDR (EU) 2017/746.
This includes Iceland, Liechtenstein, and Norway, which have implemented EU medical device and IVD regulations through the EEA Agreement. It also incorporates the EU Outermost Regions that are part of EU member states but not located inside Europe. Compliance with the MDR/IVDR is also compulsory in countries that have customs union agreements with the EU and in Northern Ireland, the latter owing to its distinct geopolitical status.
Finally, compliance with the MDR/IVDR is a possibility for Great Britain and Switzerland, both of which continue to recognize the CE mark.
Related next steps
For difficulties in navigating the EU regulatory environment or challenges in complying with horizontal EU regulations, RQm Corp+ provides expert support in developing comprehensive regulatory strategies that ensure medical devices and IVDs adhere to all relevant EU legislation.
Acknowledgments
Produced from materials originally authored by Chris A. Parr, Principal, CRO at RQM+ Corp.
References and further reading:
- European Union. EU country profiles | European Union. (online) Available at: https://european-union.europa.eu/principles-countries-history/eu-countries_en#header_countries_list.
- Cane, B. (2025). European Countries That Are Not Members Of The European Union. (online) WorldAtlas. Available at: https://www.worldatlas.com/geography/european-countries-that-are-not-members-of-the-european-union.html.
- European Commission. Inforegio - The EU and its outermost regions. (online) Available at: https://ec.europa.eu/regional_policy/policy/themes/outermost-regions_en.
- Federal Chancellery. Fedlex. (online) Available at: https://www.fedlex.admin.ch/eli/cc/2020/552/en.
- GOV.UK. (2024). Implementation of the Future Regulations. (online) Available at: https://www.gov.uk/government/publications/implementation-of-the-future-regulation-of-medical-devices/implementation-of-the-future-regulations.
- GOV.UK. Statement of policy intent: international recognition of medical devices. (online) Available at: https://www.gov.uk/government/publications/implementation-of-the-future-regulation-of-medical-devices/statement-of-policy-intent-international-recognition-of-medical-devices.
For further reading – Braving the Tariff Storm: Strategies for MedTech Companies
About RQM+
Two industry leaders - R&Q and Maetrics - merged in 2020 to become RQM+, the largest medical device and diagnostics-focused full-service regulatory and quality consulting firm in the world. The integration of our leadership teams and the extensive skills and experience of our collective resources have created a highly agile and scalable global organization.
With offices in the United States, United Kingdom, and Switzerland, our large global footprint allows us to meet the regulatory and quality support needs of international clients of all sizes.
Order an Audit
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.