This article will examine market access outside the EU/EEA based on reliance and recognition.
For countries outside the European Union (EU) and European Economic Area (EEA), regulatory reliance and recognition can be important elements of market access strategies. This article focuses on reliance and recognition based on the CE mark and reviews selected non-EU/EEA countries where manufacturers can utilize CE marking as part of their regulatory strategies to achieve market access.
What is regulatory reliance?
The “Draft Playbook for Medical Device Regulatory Reliance Programs” defines regulatory reliance as a process in which a regulatory authority in one jurisdiction considers and gives considerable weight to evaluations conducted by another regulatory authority when making its own decision.
Even when an authority relies on the decisions, assessments, and information of others, it remains independent, responsible, and accountable for the decisions taken.
What is regulatory recognition?
According to the same draft playbook, regulatory recognition refers to the acceptance of another regulator’s regulatory decision. Recognition must be grounded in evidence that the reference authority’s regulatory requirements adequately satisfy the relying authority’s regulatory standards. Recognition may be unilateral or part of a mutual recognition agreement.
The CE mark (Conformité Européene)
Although the CE mark for medical devices is primarily intended for the EU/EEA, it is also recognized or accepted in multiple countries outside these regions. The following list shows where CE-marked medical devices can potentially support market access beyond the EU/EEA (map constructed using source material from Vemaps.com):
- Europe
- Albania
- Bosnia and Herzegovina
- Montenegro
- North Macedonia
- Serbia
- Northern Ireland
- Switzerland
- Turkey
- United Kingdom
- Asia Pacific (APAC)
- Australia
- Malaysia
- Singapore
- Middle East and Africa (MEA)
The sections below take a closer look at each country (in alphabetical order).
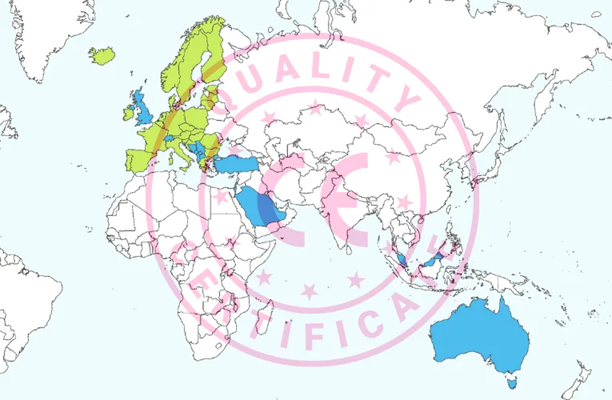
Image Credit: RQM+
Albania (Shqipëria)
Although located in Europe, Albania is not an EU or EEA member. The country is aligning its medical device regulations with the MDR/IVDR and recognizes the CE mark for market access. As a result, CE-marked medical devices and IVDs under the MDR (or IVDR) do not require a complete local conformity assessment.
The CE mark is a prerequisite for registration and market access. The responsible authority is the National Agency for Medicines and Medical Devices (Agjencia Kombëtare e Barnave dhe Pajisjeve Mjekësore, AKBPM).1
Australia
In Australia, the Therapeutic Goods Administration (TGA) permits the use of CE certificates issued under MDR/IVDR to support device approval applications.2 This forms part of a regulatory reliance approach to streamline access to safe and effective medical devices.
Manufacturers whose devices have already been evaluated by a notified body may be eligible for an abridged conformity assessment. This may involve submitting fewer documents, a reduced depth in TGA evaluation, and reductions in assessment fees.
Bosnia and Herzegovina (BiH)
Bosnia and Herzegovina (BiH), though located in Europe, is not a member of the EU or EEA. The country is aligning its regulatory framework with the MDR/IVDR and recognizes the CE mark for market access. However, local registration is mandatory prior to placing a device on the market.
The competent authority is the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina (ALMBiH).3 Every device requires registration before market placement, and products must bear CE marking under the MDR or IVDR for IVDs, as applicable for the device’s classification.
Israel
Israel is located outside Europe and is not a member of the EU or EEA. In Israel, the use of foreign market authorization evidence plays a substantial role during the medical device and IVD registration process, especially under the Fast-Track Route managed by the Ministry of Health’s AMAR (Medical Devices Division).4
Israel permits medical devices approved in specific reference countries (including the EU) to obtain registration more rapidly via the Fast-Track Route. Fast-Track registrations are valid for up to five years (three years for implants). The Israeli approval cannot exceed the validity period of the reference country’s authorization.
Malaysia
Malaysia does not automatically accept the CE mark for general market access; however, CE marking plays an important role in simplifying the conformity assessment process, particularly for medical devices and IVDs. Medical devices and IVDs approved by recognized foreign regulatory authorities (including CE-marked devices) may undergo a streamlined conformity assessment.
Malaysia’s Medical Device Authority (MDA) issued Circular Letter No. 1/2025, introducing a “verification” process that enables more efficient registration of CE-marked devices.5
Montenegro
Montenegro is located in Europe but is not a member of the EU or EEA. In Montenegro, overseas market authorization evidence - especially from the EU and other recognized jurisdictions - supports the medical device registration process.
Although Montenegro does not have a formal regulatory reliance pathway, it accepts and requires multiple documents generally issued by foreign authorities. A CE certificate under MDR or IVDR may be used to show compliance with international standards and to facilitate the local conformity assessment process with the Institut za lijekove i medicinska sredstva Crne Gore (CINMED).6
Northern Ireland
The regulation of medical devices and IVDs in Northern Ireland is distinct due to the Windsor Framework agreement, which preserves alignment with EU laws for goods. As a result, Northern Ireland continues to follow the MDR and IVDR. CE-marked devices under MDR/IVDR are mandatory and accepted for placement on the Northern Ireland market.
The MHRA acts as the competent authority for medical devices in Northern Ireland, while EU Notified Bodies carry out conformity assessments under MDR/IVDR.
North Macedonia
Despite being located in Europe, North Macedonia is not a member of the EU or EEA. Regulation of medical devices is governed by the Agency for Medicines and Medical Devices (MALMED), and current reforms aim to better match EU requirements as part of the nation’s EU accession process.7
While North Macedonia does not use a formal regulatory reliance framework like Australia or Israel, it accepts and reviews foreign market authorization evidence, especially from the EU and other regulatory authorities, during device registration. The CE certificate can streamline the evaluation process, particularly for devices already approved in the EU or other recognized jurisdictions.
Saudi Arabia
In Saudi Arabia, overseas market authorization evidence plays a supportive but not standalone role in the medical device and IVD registration process. The Saudi Food and Drug Authority (SFDA) is the regulatory authority, and every device is required to obtain Medical Device Marketing Authorization before entering the market.8
The CE certificate is generally included as part of the supportive information, but full documentation and conformity assessment are still required.
Serbia
Serbia is located in Europe but is not a member of the EU or EEA. The country recognizes foreign market authorization evidence, which can support medical device and IVD registration, although it is insufficient on its own. All devices must be registered with the Medicines and Medical Devices Agency of Serbia (ALIMS) prior to market placement.9
Serbia does not have a formal regulatory reliance pathway, but documentation issued by recognized jurisdictions (including the EU) is accepted to support the evaluation process. As part of its EU accession process, Serbia is aligning with EU standards. The CE mark is accepted for medical devices provided that the product meets Serbian technical regulatory requirements.
Singapore
In Singapore, the Health Sciences Authority (HSA) allows the use of overseas market authorization evidence to support medical device and IVD registration, particularly through its abridged evaluation routes.10 This forms part of Singapore’s internationally aligned, risk-based regulatory framework.
Singapore recognizes approvals from multiple reference regulatory agencies, including EU-notified bodies. CE-marked devices may qualify for an abridged evaluation in Singapore.
Switzerland
Switzerland is located in Europe but is not a member of the EU or EEA. Traditionally, the use of overseas market authorization evidence, particularly CE marking, has played a central role in Swiss medical device regulation.
However, due to alterations in the EU-Switzerland Mutual Recognition Agreement (MRA), the regulatory landscape has transformed, pushing Switzerland to adapt its approach to include controlled acceptance of non-EU approvals, including those from the US FDA.
Although Switzerland relies heavily on CE marking, it is planning to open up to FDA-cleared devices through a controlled pathway. This represents a move toward regulatory diversification, while maintaining stringent safety and performance requirements. Swissmedic is the national authority responsible for medical device regulation.11
Turkey (Türkiye)
Although partially located in Europe, Turkey is not a member of the EU or EEA. In Turkey, overseas market authorization evidence, particularly CE marking, plays a central role in the medical device regulatory framework due to the EU–Turkey Customs Union.
Turkey has fully harmonized its medical device regulations with the MDR and IVDR. To be placed on the Turkish market, medical devices and IVDs must carry the CE mark. CE marking is recognized as proof of conformity with Turkish regulations, which are aligned with EU directives. The Turkish Medicines and Medical Devices Agency (TMMDA) is the national authority responsible for device regulation.12
United Kingdom
In the United Kingdom, the use of foreign market authorization evidence for medical devices and IVDs is evolving under the post-Brexit regulatory framework. The UK now maintains separate systems for Great Britain (England, Scotland, and Wales) and Northern Ireland, with differing requirements for each.
Medical devices and IVDs carrying the CE mark may be placed on the Great Britain market until June 30, 2030. After this date, UKCA marking will become compulsory* unless the proposed new international reliance routes are formally implemented. The Medicines and Healthcare products Regulatory Agency (MHRA) serves as the national authority responsible for device regulation.13
The MHRA’s recent response to that previous consultation also signals an intention to progress with a reliance scheme that accommodates ‘approvals’ from comparable regulator countries (Australia, Canada and the USA).14
*Recently, the MHRA announced that it will open further consultation on whether EU CE-marked medical devices should be recognized indefinitely, following feedback from its earlier consultation on international reliance schemes.
Summary
Although the CE mark is mainly intended for the EU and EEA, several countries outside these regions acknowledge or rely on CE marking to streamline local regulatory processes. Multiple non-EU/EEA countries accept or rely on CE marking to varying degrees, and for some it is mandatory. This reliance and recognition may be unilateral (one-way acceptance) or mutual (based on formal agreements), such as mutual recognition agreements.
Manufacturers utilizing CE marking in this way should also be aware of each country’s national legislation. While CE marking may open the door, national legislation may cover aspects such as language rules, unique device identification (UDI), and post-market surveillance.
Related next steps
For difficulties in navigating the EU regulatory landscape or challenges in complying with horizontal EU legislation, RQM+ Corp’s specialized regulatory experts can assist manufacturers in developing comprehensive regulatory strategies designed to ensure that medical devices and IVDs meet all applicable EU requirements.
Acknowledgments
Produced from materials originally authored by Chris A. Parr, Principal, CRO at RQM+ Corp.
References and further reading:
- AKBPM Al. (2025). Fillimi. (online) Available at: https://akbpm.gov.al/.
- Therapeutic Goods Administration (2025). Therapeutic Goods Administration (TGA). (online) Therapeutic Goods Administration (TGA). Available at: https://www.tga.gov.au/.
- ALMBIH. (2024). Agency for Medicines Product and Medical Devices of Bosnia and Herzegovina. (online) Available at: https://almbih.gov.ba/en/agencija-za-lijekove-i-medicinska-sredstva-bosne-i-hercegovine-eng/.
- Medical Device Division, Ministry of Health
- Medical Device Authority. Official Portal of Medical Device Authority (MDA) Malaysia - Medical Device Authority (MDA). (online) Available at: https://www.mda.gov.my/.
- Cinmed. (2025). Home - Cinmed. (online) Available at: https://cinmed.me/en/.
- Malmed. Malmed |. (online) Available at: https://malmed.gov.mk/.
- Saudi Food and Drug Authority. Saudi Food and Drug Authority. (online) Available at: https://www.sfda.gov.sa/en.
- ALIMS. (2024). Home page. (online) ALIMS. Available at: https://www.alims.gov.rs/english/.
- HSA. Home. (online) Available at: https://www.hsa.gov.sg/.
- Copyright Swissmedic 2019 (2019). Swissmedic. (online) Swissmedic.ch. Available at: https://www.swissmedic.ch/swissmedic/de/home.html.
- Turkish Medicines and Medical Devices Agency . Titck - Turkish Medicines and Medical Devices Agency. (online) Available at: https://www.titck.gov.tr/.
- MHRA (2025). Medicines and Healthcare products Regulatory Agency. (online) GOV.UK. Available at: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency.
- GOV.UK. (2025). Response to international reliance, UKCA marking and in vitro diagnostic devices consultation proposal. (online) GOV.UK. Available at: https://www.gov.uk/government/consultations/consultation-on-medical-devices-regulations-routes-to-market-and-in-vitro-diagnostic-devices/outcome/response-to-international-reliance-ukca-marking-and-in-vitro-diagnostic-devices-consultation-proposal.
About RQM+
Two industry leaders - R&Q and Maetrics - merged in 2020 to become RQM+, the largest medical device and diagnostics-focused full-service regulatory and quality consulting firm in the world. The integration of our leadership teams and the extensive skills and experience of our collective resources have created a highly agile and scalable global organization.
With offices in the United States, United Kingdom, and Switzerland, our large global footprint allows us to meet the regulatory and quality support needs of international clients of all sizes.
Order an Audit
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.