More than 120 peer-reviewed cell types have already been successfully cryopreserved using Bambanker™, all consolidated in one place for easy access and reference.
Background
Cryopreservation is central to how most labs manage their cell workflows today. By pairing a cryoprotectant with controlled cooling and ultra-low-temperature storage, researchers can effectively pause cellular activity while maintaining important phenotypes so experiments can be resumed later with confidence and continuity.
With increasing pressure to improve study reproducibility and a growing need to store and share well-characterized cells (from standard cell lines to more advanced programs), many researchers are making standardized, publication-supported freezing conditions a priority. To make that evidence easy to verify, a DOI-linked overview brings together cell types reported in peer-reviewed studies as cryopreserved with Bambanker™.
Cell type matters
The type of cell you’re working with makes a real difference in cryopreservation, because not all cells handle freezing stress the same way. Factors such as membrane composition, cell size, cytoplasmic water content, and sensitivity to osmotic shifts all shape how a cell responds to cryoprotectants, cooling rates, and thawing conditions.
Robust immortalized cell lines often recover reliably across a range of parameters. In contrast, primary cells and stem cells (including induced pluripotent stem cells, or iPSCs) can be far less forgiving: small changes in handling time, cell density, or post-thaw recovery conditions can affect viability, attachment, growth kinetics, and even phenotype.
That’s why examples that are both cell-type-specific and backed by literature are so valuable. They reduce the need for trial and error by showing how comparable cells have been cryopreserved and recovered in practice.
Common cryopreservation pitfalls (and why results can vary)
Even within the same cell type, slight variations in handling can result in significantly different outcomes after thawing, particularly for sensitive primary cells and stem cells.
Common sources of variability include freezing-stressed or over-confluent cultures, inconsistent cell density, prolonged exposure time after adding cryoprotectant at room temperature, differences in cooling consistency, and post-thaw recovery steps, such as dilution timing and initial culture conditions. Standardizing these fundamentals reduces variation between batches and users and improves comparability across experiments.
Because these variables can vary from one lab to another, and even between closely related cell types, having published examples to refer to makes a real difference. They offer a practical starting point, help reduce uncertainty, and give you a clearer sense of what has already worked under comparable conditions.
The DOI-linked article below brings together peer-reviewed reports of cell types cryopreserved with Bambanker™, so you can quickly find relevant references and see how others have approached similar workflows.
 Image Credit: Holo Art/Shutterstock.com
Image Credit: Holo Art/Shutterstock.com
A simplified approach
Bambanker™ is a ready-to-use cryoprotectant designed to streamline mammalian cell freezing by:
- Eliminating serum requirements
- Reducing hands-on steps
- Standardizing freezing conditions
- Supporting reproducible results across labs
Its performance has been documented in over 120 peer-reviewed publications, spanning a diverse range of cell and sample types.
What the full list includes
- A curated table of 120+ cell types and samples cryopreserved using Bambanker™
- Direct DOI links to the original peer-reviewed studies
- One representative publication per cell or sample type to keep the table concise and practical
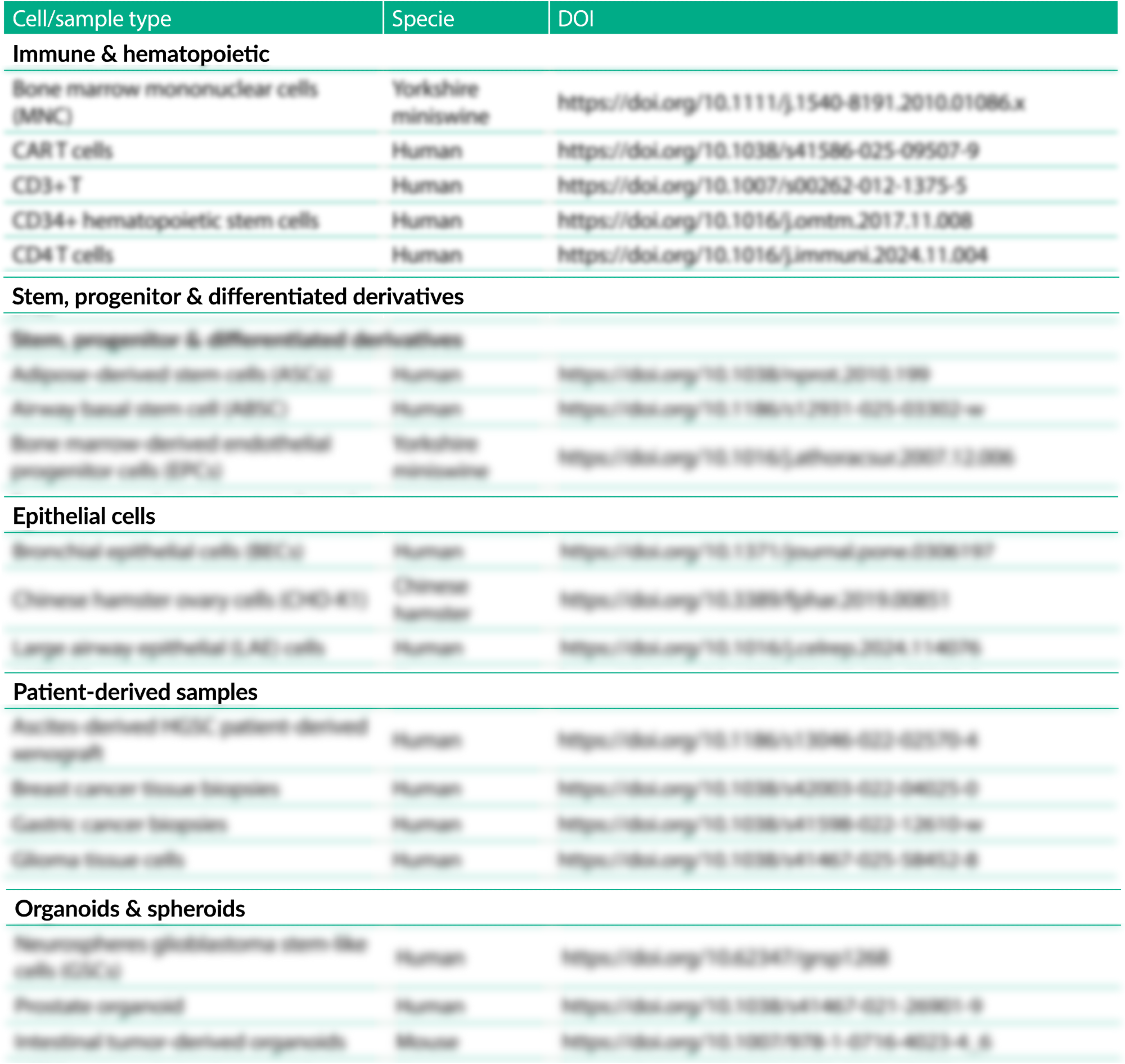 Image Credit: Nippon Genetics
Image Credit: Nippon Genetics
Update policy
This table is reviewed and updated annually to incorporate newly published data. The current version includes literature published up to December 2025.
About NIPPON Genetics EUROPE GmbH
Our contribution to your success – We are committed to ensuring that you can achieve your goals!
NIPPON Genetics EUROPE is a global biotech company founded in 2005 with its own research, development and production based in Germany. By providing an extensive range of selected and groundbreaking products for the life science sector, we would like to be a reliable partner and contribute to achieving our customers' individual goals. The majority of our employees have a scientific background because we want to understand which problem solutions our customers care about.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.