2. What should I know before I use Entyvio?
Do not use if you have ever had an allergic reaction to vedolizumab or any of the
ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
are pregnant or plan to become pregnant or are breastfeeding or plan to breastfeed.
3. What if I am taking other medicines?
Some medicines may interfere with Entyvio and affect how it works.
4. How do I use Entyvio pre-filled pen?
Entyvio solution for injection is given by injection just under the skin (subcutaneous
injection).
5. What should I know while using Entyvio?
|
Things you should do
|
Read this leaflet carefully before you start using this medicine. It contains important
information for you.
Keep this leaflet. You may need to read it again.
If you have any questions, ask your doctor, pharmacist or nurse.
|
|
Things you should not do
|
Do not stop using Entyvio without checking with your doctor.
Do not give this medicine to anyone else, even if they have the same condition as
you.
|
|
Driving or using machines
|
Be careful driving or operating machinery until you know how Entyvio affects you.
If you feel dizzy, do not drive or use tools or machines.
|
|
Looking after your medicine
|
Store in a refrigerator at 2°C to 8°C. Do not freeze.
Keep the pre-filled pens in the original box to protect from light.
If needed, a single pre-filled pen can be left out of the refrigerator protected from
light at room temperature (up to 25 °C) for up to 7 days. Discard the pre-filled pen
if not used within the 7 days of room temperature storage.
|
6. Are there any side effects?
Very common: common cold, joint pain, headache. Common: fever, flu (influenza), nose
or throat infection, bronchitis, chest infection, cough, throat pain, nausea, itching,
rash and redness, pain in the extremities, back pain, tiredness, injection site reactions
(including pain, swelling, redness or itching. Serious side effects: allergic reactions,
infections, liver injury.
Active ingredient(s):
vedolizumab
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using Entyvio pre-filled pen for
subcutaneous injection. It does not take the place of talking to your doctor or pharmacist.
You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using Entyvio pre-filled pen.
The information in this leaflet was last updated on the date listed on the final page.
More recent information on the medicine may be available. You should ensure that you
speak to your pharmacist or doctor to obtain the most up to date information on this
medicine. You can also download the most up to date leaflet from www.takeda.com/en-au.
Those updates may contain important information about the medicine and its use of
which you should be aware.
Where to find information in this leaflet:
1. Why am I using Entyvio?
Entyvio pre-filled pen contains the active ingredient vedolizumab.
Vedolizumab is a monoclonal antibody. Monoclonal antibodies are proteins that recognise
and bind to certain special proteins in the body.
Entyvio specifically binds to a protein called integrin α4β7 present on certain white
blood cells. Integrin α4β7 can act to increase inflammation seen in ulcerative colitis
and Crohn’s disease. Entyvio works by blocking α4β7 integrins and so reduces inflammation.
Ulcerative colitis
Ulcerative colitis is an inflammatory disease of the large bowel. Entyvio is used
to treat the signs and symptoms of moderate to severe ulcerative colitis in adults
who have not responded well enough or are intolerant to other treatments.
Crohn’s disease
Crohn’s disease is an inflammatory disease of the bowel. It may also affect any part
of the gut. Entyvio is used to treat the signs and symptoms of moderate to severe
Crohn’s disease in adults who have not responded well enough or are intolerant to
other treatments.
2. What should I know before I use Entyvio?
Warnings
Do not use Entyvio if:
You are allergic to vedolizumab, or any of the ingredients listed at the end of this
leaflet.
Symptoms of an allergic reaction may include wheezing or difficulty breathing, hives,
itching of the skin, swelling or dizziness.
You have an active severe infection, such as for example tuberculosis, blood poisoning,
serious abscesses.
After the expiry date printed on the pack or if the packaging is damaged or shows
signs of tampering. If it has expired or is damaged return it to your pharmacist for
disposal.
Check with your doctor if you:
Have an infection, or think you have an infection.
Are going to receive any vaccination or have recently had a vaccination. Entyvio may
affect the way you respond to a vaccination.
Have any allergies to any other medicines, foods, preservatives or dyes.
Take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or intend to become pregnant.
The effects of Entyvio in pregnant women are not known. Therefore, this medicine is
not recommended for use during pregnancy unless you and your doctor decide that the
benefit to you clearly outweighs the potential risk to your baby. Your doctor can
discuss the risks and benefits involved.
Tell your doctor if you are breastfeeding or intend to breastfeed.
Entyvio passes into breast milk and it is not known what effect this may have on your
baby.
Women who are breastfeeding or intend to breastfeed should talk to their doctor about
whether or not to use Entyvio.
Use in Children
There is not enough information to recommend the use of this medicine for children
under the age of 18 years.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking or have recently taken any other
medicines, including any medicines, vitamins or supplements that you buy without a
prescription from your pharmacy, supermarket or health food shop.
Entyvio should not be given with other biologic medicines that suppress your immune
system as this combination has not been studied in clinical trials.
Tell your doctor if you have previously taken natalizumab (a medicine for multiple
sclerosis) or rituximab (a medicine for certain types of cancer and rheumatoid arthritis).
Your doctor will decide if you can use Entyvio.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect Entyvio.
4. How do I use Entyvio pre-filled pen?
How much to use
Always use Entyvio exactly how your doctor or nurse has told you. Check with your
doctor, nurse or pharmacist if you are not sure.
Treatment with Entyvio is the same for ulcerative colitis and Crohn’s disease.
When starting treatment, the initial doses of Entyvio will be given to you by your
doctor or nurse in a hospital or a clinic, through a drip in one of the veins in your
arm (intravenous infusion) over about 30 minutes.
After at least 2 intravenous infusions, you can start using Entyvio by an injection
under the skin (subcutaneously).
The first subcutaneous dose is administered at the time of the next scheduled intravenous
infusion, and every 2 weeks thereafter.
Follow the instructions provided and use Entyvio pre-filled pen until your doctor
tells you to stop.
The recommended dose is 108 mg of Entyvio (1 pre-filled pen) administered by subcutaneous
injection once every 2 weeks.
The subcutaneous injections can be given by yourself or a caregiver, after appropriate
training on how to do it.
How to use Entyvio pre-filled pen
Your Entyvio single-dose pre-filled pen:
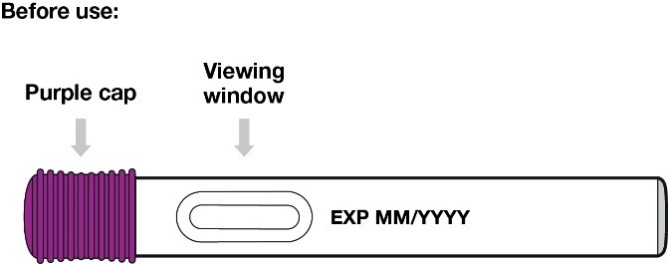
1.
Place what you need for the injection on a clean, flat surface
Take the pre-filled pen box from the refrigerator.
If you are opening the carton for the first time, check to make sure the carton is
properly sealed. Do not use the pre-filled pen(s) if any of the seals on the box are broken or missing.
Check the expiry date on the box. Do not use if the expiry date on the box has passed.
Remove one pre-filled pen from the carton. Keep any remaining pre-filled pens in the
carton in the refrigerator.
Wait
30 minutes to let the pre-filled pen come to room temperature (Figure 1).
Do not warm the pre-filled pen in any other way.
Do not let it sit in direct sunlight.
Do not take the pre-filled pen out of its tray until you are ready to inject.

Figure 1
Gather the supplies that are not in the box (Figure 2):
Alcohol swab
Cotton ball or gauze
Sharps disposal container

Figure 2
2.
Inspect the pre-filled pen
Wash your hands (Figure 3).
Figure 3
Peel back the paper on the tray and lift the pre-filled pen out (Figure 4).
Figure 4
Inspect the pre-filled pen for damage.
Do not use the pre-filled pen if any part of it is damaged.
Check the expiry date on the pre-filled pen (Figure 5).
Do not use if the expiry date on the pre-filled pen has passed.
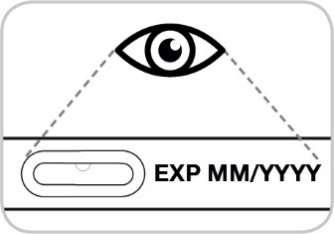
Figure 5
Check the medicine in the viewing window. It should be colourless to yellow.
Do not use the pre-filled pen if the medicine is cloudy or has particles floating in it.
You may see air bubbles in the pre-filled pen. This is normal.
Do not attempt to remove air bubbles from the pre-filled pen.
Do not shake.
3.
Prepare the injection site
Choose an injection site on your bare skin from 1 of the following (Figure 6).
Front of the thighs, or
Stomach area (abdomen) except for the area 5 cm around the belly button (navel), or
Back of the upper arm (only if a caregiver gives the injection).
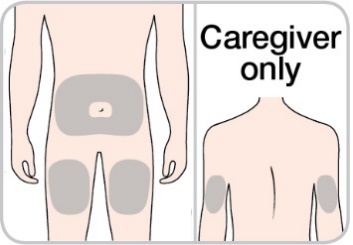
Figure 6
Use a new injection site for each injection. If you want to use the same injection
site, make sure it is not the same spot you used for the last injection.
Do not inject into moles, scars, bruises, or skin that is tender, hard, red, or damaged.
Wipe the chosen site with an alcohol swab. Let your skin dry (Figure 7).
Do not touch this area again before you inject.
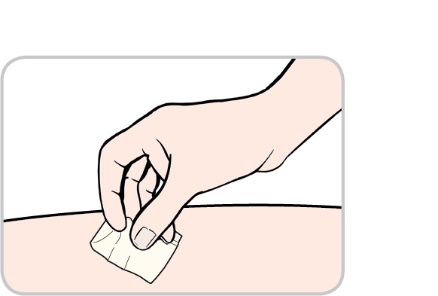
Figure 7
Pull the purple cap straight off and throw it away (Figure 8).
Do not put or press thumb, fingers or hand over the yellow needle shield.
Do not re-cap the pre-filled pen.
Do not use a dropped pre-filled pen.
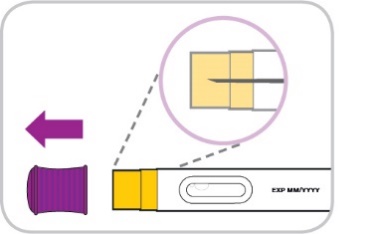
Figure 8
Hold the pre-filled pen so you can see the viewing window.
Place the pre-filled pen at
90 degrees to the injection site.
Be sure the yellow end is toward the injection site.
Do not push down until you are ready to inject.
Push down on the pre-filled pen as far as it will go to begin the injection (Figure 9).
Hold and count to 10 while pushing down with constant pressure (Figure 10). This will allow all of the
medicine to be injected.
You may hear 2 clicks, one at the start and one near the end of the injection.
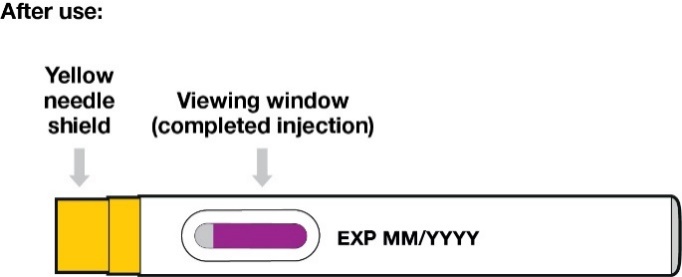
Confirm that the viewing window is filled with purple before you stop pushing (Figure 11).
You will see a small amount of grey in the window. This is normal.
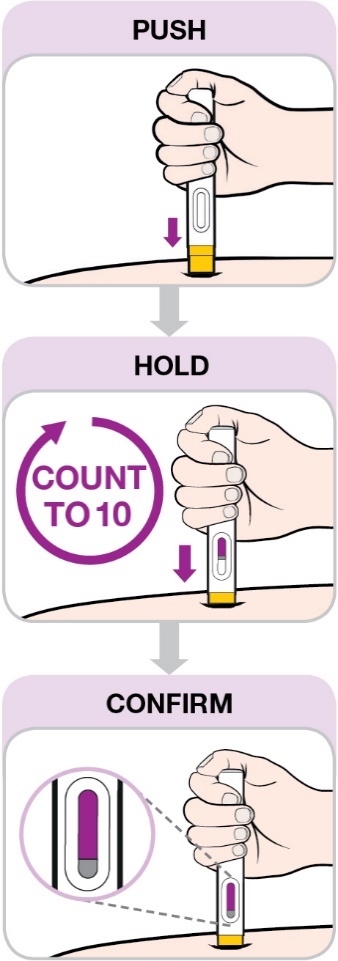
Figures 9, 10 and 11
Lift the pre-filled pen from the injection site.
The yellow needle shield will drop down and lock over the needle.
If the viewing window did not fill completely, call your doctor, nurse or pharmacist.
You may not have received your full dose of medicine.
You may see a small amount of blood at the injection site. If you do, press on your
skin with a cotton ball or gauze.
5.
Throw away used materials
Put the used pre-filled pen in the sharps container, immediately after use (Figure
12).
Do not recycle or throw away the pre-filled
pen in your household trash.
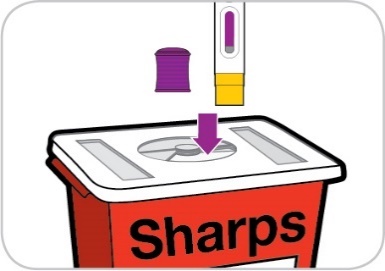
Figure 12
The rest of the material can be thrown in your household rubbish.
Always keep the Entyvio pre-filled pens and the sharps disposal container out of the
reach of children.
If you do not understand the instructions provided with this medicine, ask your doctor
or pharmacist for help.
How long to use it for
Continue using your medicine for as long as your doctor tells you.
You should not stop using Entyvio without talking with your doctor first.
If you forget to use Entyvio pre-filled pen
If you forget or miss a dose, the next dose should be injected as soon as possible
and then every 2 weeks thereafter.
Do not take a double dose to make up for the dose you missed.
If you use too much Entyvio
If you think that you have used too much Entyvio, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Entyvio?
Things you should do
When you first receive this medicine and during the course of the treatment, also
between doses, tell your doctor or pharmacist immediately if you:
Have an infection, or think you have an infection, such as if you develop chills, shivering, persistent
cough or a high fever. Some infections may become serious and possibly even life-threatening
if left untreated.
Experience signs of an allergic reaction or other reaction to the infusion such as wheezing, difficulty breathing, hives,
itching, swelling or dizziness.
Are going to receive any vaccination or have recently had a vaccination. Entyvio may affect the way you respond to a vaccination.
If you are about to be started on any new medicine, remind your doctor that you are
using Entyvio.
If you become pregnant while you are taking this medicine, tell your doctor immediately.
Call your doctor immediately if you:
Experience blurred, loss of or double vision, difficulty speaking, weakness in an
arm or a leg, a change in the way you walk or problems with your balance, persistent
numbness, decreased sensation or loss of sensation, or memory loss or confusion. These
may all be symptoms of a serious and potentially fatal brain condition known as progressive multifocal leukoencephalopathy (PML).
Things you should not do
Do not use this medicine to treat any other complaints unless your doctor tells you
to.
Do not give this medicine to anyone else, even if they have the same condition as
you.
Do not stop using Entyvio without checking with your doctor.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Entyvio
affects you.
This medicine may have a minor influence on your ability to drive or use tools or
machines. A small number of patients have felt dizzy after receiving Entyvio.
If you feel dizzy, do not drive or use tools or machines.
Looking after your medicine
Store in a refrigerator at 2°C to 8°C. Do not freeze.
Keep the pre-filled pen(s) in the original box to protect from light.
If needed, one pre-filled pen can be left out of the refrigerator protected from light
at room temperature (up to 25 °C) for up to 7 days. Discard the pre-filled pen if
not used within the 7 days of room temperature storage.
Do not use this medicine if you notice any particles in the liquid or discolouration
(should be colourless to yellow) prior to administration.
Do not use this medicine after the expiry date.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on windowsills.
Keep it where young children cannot reach it.
When to discard your medicine
Entyvio pre-filled pen is for single use only and must be discarded after the injection.
Dispose of the pens in a sharps container.
Ask your doctor or pharmacist for information about where you can get a sharps container
to safely dispose of your used pens, if you do not have one. Dispose of the full container
as instructed by your doctor or pharmacist.
Do not put the used pens in your normal household rubbish.
Getting rid of any unwanted medicine
If your doctor tells you to stop using Entyvio, or the product has passed its expiry
date, take it to any pharmacy for safe disposal.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
Do not be alarmed by this list of possible side effects. You may not experience any
of them.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Entyvio contains
|
Active ingredient
(main ingredient)
|
vedolizumab
Each pre-filled pen contains 108 mg of vedolizumab.
|
|
Other ingredients
(inactive ingredients)
|
citric acid monohydrate
sodium citrate dihydrate
histidine
histidine hydrochloride monohydrate
arginine hydrochloride
polysorbate 80
sterile water for injections
|
Do not use this medicine if you are allergic to any of these ingredients.
What Entyvio pre-filled pen looks like
Entyvio solution for injection is a colourless to yellow solution for injection provided
in a glass pre-filled pen equipped with an automated needle shield to extend and lock
over the needle once the device is removed from the injection site.
Entyvio is available in boxes containing 1, 2 or 6 pre-filled pen(s) (AUST R 317262).
Not all pack sizes may be marketed.
Who distributes Entyvio
Takeda Pharmaceuticals Australia Pty Ltd
Level 39
225 George Street
Sydney, NSW 2000
Australia
Tel: 1800 012 612
This leaflet was prepared in July 2025.
ENTYVIO® and the ENTYVIO Logo® are registered trademarks of Millennium Pharmaceuticals, Inc., a Takeda company. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.