Introduction
What is orforglipron?
How it works
Clinical evidence
Comparison with other GLP-1s
Market outlook
References
Further reading
Orforglipron, the first promising oral small-molecule GLP-1, is demonstrating weight-loss and metabolic results that could reshape obesity treatment and challenge injectable standards.
 Image Credit: DukiPH / Shutterstock.com
Image Credit: DukiPH / Shutterstock.com
Introduction
Orforglipron is an orally bioavailable next-generation glucagon-like peptide 1 (GLP-1) receptor agonist (GLP-1RA) that has emerged as a potential alternative to injectable agents like semaglutide based on early Phase 1, 2, and 3 clinical trials demonstrating glucose-lowering and weight-loss efficacy without the need for injection-based delivery.2,3,5
What is orforglipron?
Orforglipron (LY3502970) is a small-molecule, non-peptide GLP-1RA designed for oral use. Compared to popular GLP-1RAs like semaglutide, orforglipron does not require injection or an absorption enhancer with fasting to achieve exposure, and it can be taken without water or food restrictions, according to Phase 1 pharmacokinetic data.2
Cryogenic electron microscopy (CEM) imaging demonstrates that orforglipron binds within the upper helical bundle of GLP-1R and acts as a highly potent partial agonist that favors cyclic adenosine monophosphate (cAMP) signaling over beta-arrestin recruitment. The long elimination half-life of 25-68 hours further supports convenient once-daily dosing.2,3
In a Phase I multiple-dose clinical trial conducted in healthy adults, pharmacokinetics were dose-proportional, accompanied by reductions in both fasting and oral glucose tolerance glucose levels. Orforglipron treatment was also associated with slower gastric emptying time and short-term weight loss, all of which are consistent with drug class effects, with up to 5.4 kg loss over 4 weeks in higher-dose cohorts.2
Since it is non-peptide, orforglipron avoids GI degradation barriers that limit peptides, thereby enabling once-daily administration while maintaining efficacy on glucose control, body weight, and tolerability.2,3
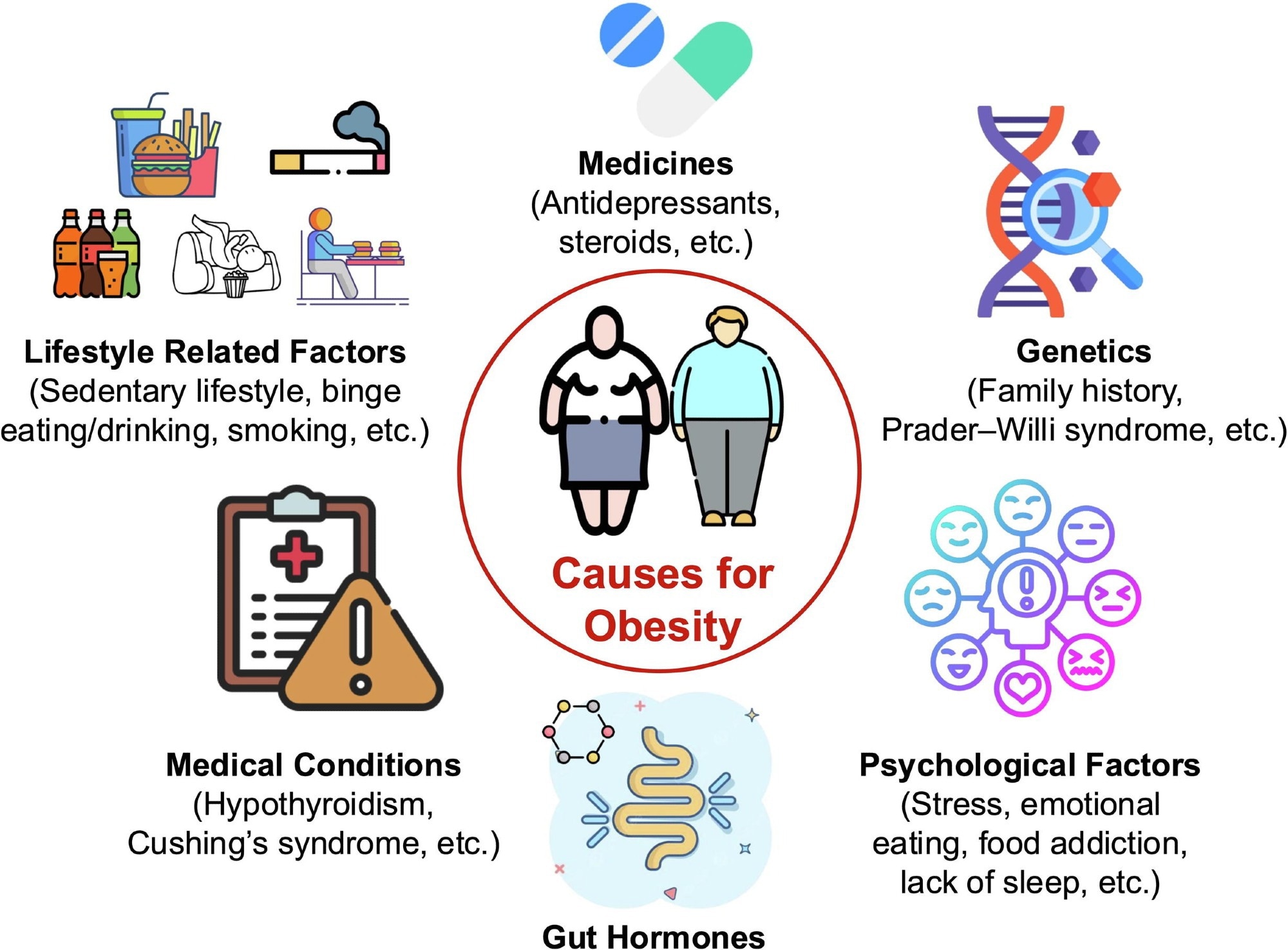
Obesity is a complex, multifactorial condition influenced by various interrelated factors, including lifestyle, medications, genetics, medical conditions, gut hormones and psychological aspects. This illustration emphasizes the intricate nature of obesity and highlights the necessity for comprehensive strategies in treatment and prevention.1
How it works
The design of orforglipron emphasizes stability in the GI tract, which allows oral dosing while preserving target engagement. Like other GLP-1 pathway drugs, orforglipron interacts with peripheral and central signaling to regulate appetite and metabolism, including delayed gastric emptying and improved post-prandial control.4
Mechanistically, binding to GLP-1R activates adenylate cyclase (AC), subsequently increasing cAMP to trigger both protein kinase A (PKA) and exchange protein directly activated by cAMP 2 (Epac2). PKA closes adenosine triphosphate (ATP)-dependent potassium (KATP) channels, depolarizes β-cell membranes, and opens voltage-dependent calcium channels.
Moreover, Epac2 engages Ras-related protein 1 (Rap1) and phospholipase C (PLC), thereby elevating inositol triphosphate (IP3) and diacylglycerol (DAG) levels. The resulting calcium influx and release lead to insulin granule exocytosis, which is supported by mitochondrial ATP synthesis.
Simultaneously, GLP-1 signaling suppresses pancreatic α-cell glucagon in a glucose-dependent manner, while gut-brain vagal pathways slow gastric emptying and enhance satiety. Together, the effects of stimulating insulin, reducing glucagon, and delaying gastric emptying improve glycemic control, resulting in a favorable hypoglycemia profile.4
Clinical evidence
In adults with obesity, 72 weeks of orforglipron treatment resulted in greater body-weight reductions than placebo, up to -11.2%, compared to -2.1% with placebo. Waist circumference, systolic blood pressure (BP), triglycerides, and non-high-density lipoprotein cholesterol (non-HDL-C) improved significantly, indicating broad cardiometabolic improvements. However, the pivotal 36-week Phase 2 trial showed a larger range of mean loss, from −9.4% to −14.7%, across doses, confirming robust dose-dependent effects.5
GI events like nausea, diarrhea, constipation, vomiting, and dyspepsia were the most frequently reported adverse effects; however, these symptoms were mild to moderate in their intensity and were most often reported during dose escalation. Treatment discontinuation due to gastrointestinal events occurred in a minority of patients, and overall, serious adverse events were similar to those with the placebo.3,5
In early T2DM managed with lifestyle alone, orforglipron lowered glycated hemoglobin A1c (HbA1c) by -1.24 to -1.48 percentage points over 40 weeks as compared to -0.41 with placebo, with no severe hypoglycemia reported and weight reduction ranging from −4.5% to −7.6%,3 which led to mean HbA1c levels of 6.5-6.7%.
Comparison with other GLP-1s
As compared to other GLP-1RAs, orforglipron is a direct and orally active small molecule, rather than a peptide. In Phase II clinical trials in adults with overweight or obesity, once-daily orforglipron led to 9-15% mean weight loss at 36 weeks, with a GI tolerability profile typical of the class.3
By contrast, oral semaglutide (Rybelsus) is a peptide that requires the absorption enhancer sodium N-(8-[2-hydroxybenzoyl]amino) caprylate (SNAC) to cross the gastrointestinal barrier and achieve bioavailability. This drug must be taken while fasting with specific intake instructions to optimize uptake, whereas orforglipron does not require fasting or enhancer co-administration.2
Mechanism differences imply greater formulation simplicity and chemical stability for orforglipron, which suggests lower production costs as compared to peptide injectables or enhancer-dependent oral peptides. These potential advantages are class-level expectations for small-molecule GLP-1RAs, rather than established price outcomes.3,5
Whereas injectable semaglutide achieved 15% mean weight loss at 68 weeks in the Semaglutide Treatment Effect in People with obesity (STEP-1) study, it requires subcutaneous administration. Comparatively, orforglipron offers once-daily oral convenience that may favor adherence, though direct head-to-head trials have not yet been conducted.5
Market outlook
Eli Lilly’s oral non-peptide GLP-1RA orforglipron is advancing through the ACHIEVE Phase III program, with Lilly signaling regulatory submissions for obesity in 2025 and type 2 diabetes in 2026, following positive results from both Phase 2 obesity (GZGI) and Phase 3 T2DM (ACHIEVE-1) trials.3,5 Pfizer also announced the development of danuglipron; however, this program was discontinued in April 2025 following a potential drug-induced liver injury signal and earlier tolerability concerns, leaving orforglipron as the leading oral small-molecule GLP-1RA in late-stage development.1
If approved, an effective once-daily oral GLP-1 could significantly expand access by lowering delivery barriers, improving adherence for injection-averse patients, and alleviating distribution constraints often associated with injectable formulations. Emerging reviews of the GLP-1 market anticipate continued demand, pipeline diversification, and health-system impact as agents move earlier in care pathways, reinforcing the case for meaningful market expansion through 2026-2028.3,4,5
References
- Gangwal, A. & Lavecchia, A. (2025). Artificial intelligence in anti-obesity drug discovery: unlocking next-generation therapeutics. Drug Discovery Today 30(4). DOI:10.1016/j.drudis.2025.104333, https://www.sciencedirect.com/science/article/pii/S1359644625000467
- Pratt, E., Ma, X., Liu, R., et al. (2023). Orforglipron (LY3502970), a novel, oral non‐peptide glucagon‐like peptide‐1 receptor agonist: a Phase 1a, blinded, placebo‐controlled, randomized, single‐and multiple‐ascending‐dose study in healthy participants. Diabetes, Obesity and Metabolism 25(9); 2634-2641. DOI:10.1111/dom.15184, https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.15184
- Rosenstock, J., Hsia, S., Nevarez Ruiz, L., et al. (2025). Orforglipron, an oral small-molecule GLP-1 receptor agonist, in early type 2 diabetes. New England Journal of Medicine 393(11). DOI:10.1056/NEJMoa2505669, https://www.nejm.org/doi/10.1056/NEJMoa2505669
- Zhang, J., Wei, J., Lai, W., et al. (2025). Focus on Glucagon-like Peptide-1 Target: Drugs Approved or Designed to Treat Obesity. International Journal of Molecular Sciences 26(4). DOI:10.3390/ijms26041651, https://www.mdpi.com/1422-0067/26/4/1651
- Wharton, S., Blevins, T., Connery, L., et al. (2023). Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. New England Journal of Medicine 389(10); 877-888. DOI:10.1056/NEJMoa2302392, https://www.nejm.org/doi/10.1056/NEJMoa2302392
Further Reading
Last Updated: Nov 17, 2025