Introduction
What is GLP-1?
Challenges of oral delivery
Overcoming barriers
Clinical outcomes
The future of oral incretins
References
Further reading
This article explains how oral GLP-1 receptor agonists achieve weight loss by overcoming gastrointestinal barriers through innovative delivery technologies and small-molecule design. It also explores future directions in oral incretin therapies, including multi-agonists and scalable, needle-free treatment strategies.
 Image Credit: lucigerma / Shutterstock.com
Image Credit: lucigerma / Shutterstock.com
Introduction
This article summarizes how oral glucagon-like peptide-1 (GLP-1) receptor agonists address weight-loss challenges, improve absorption, and enhance tolerability, as well as the promising future of GLP-1/glucose-dependent insulinotropic polypeptide (GIP) dual therapies.
What is GLP-1?
GLP-1 is a peptide hormone generated from proglucagon that is secreted after meals by enteroendocrine L-cells in the distal jejunum, ileum, and colon. GLP-1 is also produced by pancreatic α-cells and neurons in the nucleus of the solitary tract.2
As an incretin, GLP-1 regulates post-prandial glucose levels; however, it is rapidly degraded in circulation by dipeptidyl peptidase 4 (DPP-4). Binding to the GLP-1 receptor (GLP-1R), a G protein-coupled receptor (GPCR) widely expressed throughout the body, GLP-1 enhances glucose-dependent insulin secretion and suppresses glucagon, thereby supporting stable glycemia. These actions are complemented by central nervous system (CNS) effects on appetite and satiety signaling.2
Within the gastrointestinal (GI) tract, activation of GLP-1R reduces gastric muscle contractions and slows gastric emptying, prolonging satiety and reducing post-prandial glucose spikes. GLP-1 acts locally in the GI tract and via the vagus nerve, a key autonomic pathway that, when activated, further slows stomach emptying. The resulting longer gastric retention enhances satiety, reduces overall food intake, and contributes to weight management.2
GLP-1 signaling within hypothalamic circuits of the brain activates satiety-promoting neurons and inhibits hunger-promoting neurons. Together, these endocrine, neural, and GI effects demonstrate how GLP-1 is involved in blood-glucose regulation, satiety, and gastric emptying.2
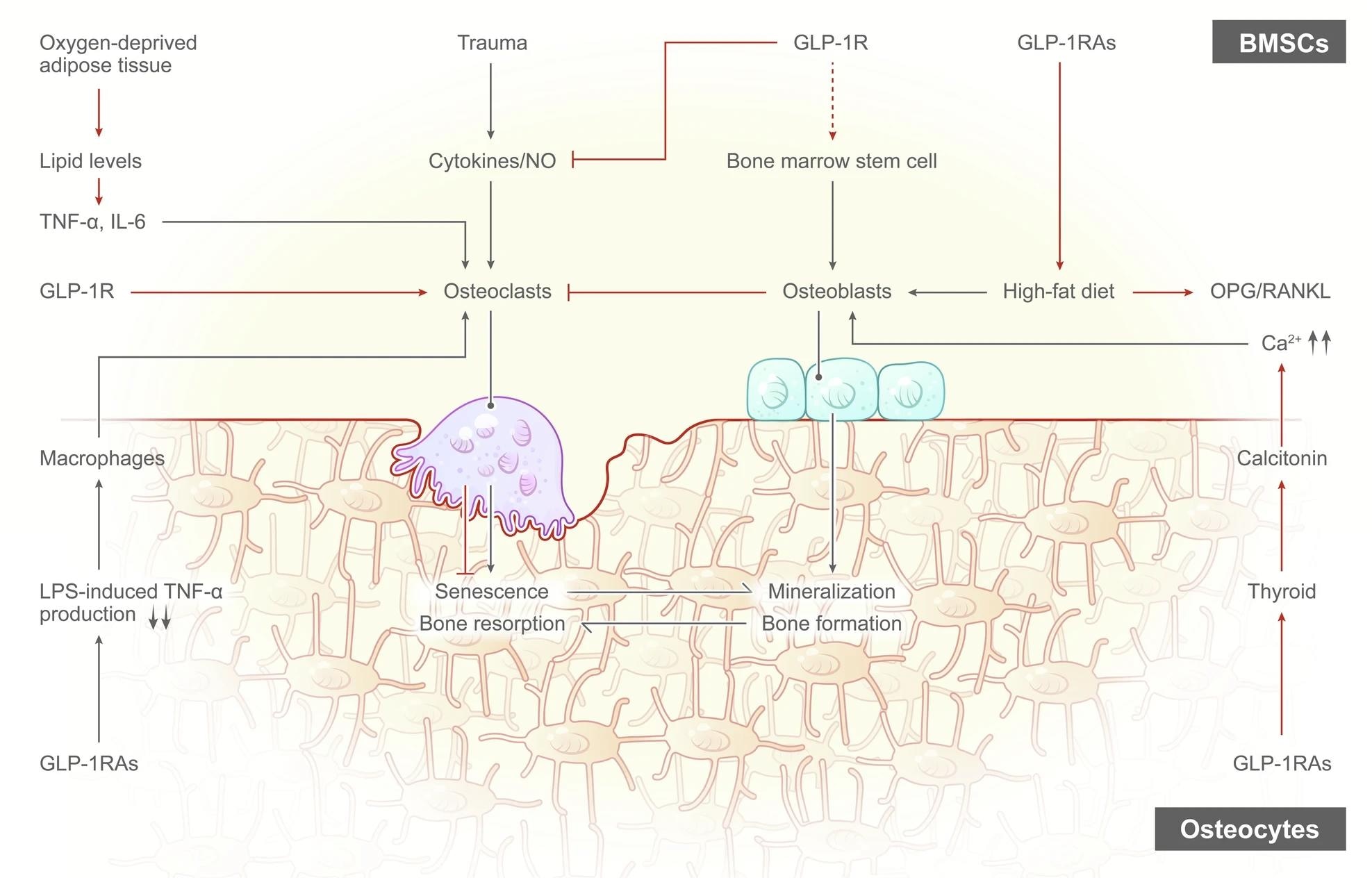
The Effects of GLP-1RAs on osteoclasts and osteoblasts. GLP-1RAs aid in weight loss by regulating the gut-brain axis and interacting with leptin, while weight loss can alleviate the harmful effects of obesity on the body, particularly in knee OA, by reducing joint loading and inflammation. Obesity disrupts bone metabolism and leads to increased bone resorption, but GLP-1RAs can inhibit this damage and improve bone health by increasing the OPG/RANKL ratio, reducing osteoclast activity, and promoting bone formation.2
Challenges of oral delivery
The low pH of 1.5-3.5 in the stomach unfolds many proteins and activates pepsin, which rapidly cleaves peptide bonds. Within the small intestine, pancreatic enzymes, including trypsin, chymotrypsin, elastase, and carboxy-/aminopeptidases, continue proteolysis, which reduces peptides to fragments or amino acids.
Protective mucus in the GI tract consists of a sticky, negatively charged mesh that slows the diffusion of large, hydrophilic macromolecules while continuously turning over, thereby pulling material away from the epithelium. Even if fragments reach the surface, epithelial tight junctions (TJs) restrict the paracellular route to tiny pores. Transcellular passage is also minimal, as peptides exceed Lipinski’s Rule of Five (Ro5) limits and carry many hydrogen-bond donors/acceptors, both of which further contribute to extremely low oral bioavailability.3
Strategies like enteric coatings to bypass gastric acid, local pH modulation and enzyme inhibitors to deactivate intestinal proteases, muco-penetrating or muco-adhesive carriers, and transient permeation enhancers to increase membrane fluidity are used to mitigate these barriers. However, variability in GI physiology and safety constraints limit universal success.
Will This New Oral GLP-1 Pill Replace Ozempic and Wegovy for Weight Loss? Dr. Decotiis Explains
Overcoming barriers
Oral delivery of GLP-1 therapies is advancing through two complementary strategies that aim to overcome low gastric stability and poor epithelial uptake. For example, oral semaglutide is co-formulated with SNAC, an absorption enhancer that locally increases gastric pH to protect the peptide from pepsin and acid, thereby promoting monomer formation. By enabling transcellular uptake across the gastric epithelium, this formulation successfully overcomes degradation and permeability barriers without the need for injections.4
Non-peptidic small-molecule GLP-1R agonists, such as orforglipron, and other investigational agents are intrinsically resistant to proteolysis and do not require permeation enhancers or fasting administration. These agents bind within deep transmembrane pockets of the receptor, often exhibit stimulatory G protein alpha subunit (Gαs)-biased signaling that can limit β-arrestin-driven internalization, and are designed for food-independent, once-daily dosing. Unlike oral semaglutide, which still requires fasting administration and SNAC to enable gastric uptake, orforglipron is absorbed without specific timing restrictions, offering practical advantages for adherence.5
However, not all small-molecule GLP-1R agonists have advanced successfully; danuglipron and lotiglipron were discontinued in development due to hepatotoxicity signals, underscoring safety considerations unique to this drug class.5
Together, SNAC-enabled peptide delivery and small-molecule agonists have the potential to expand access to incretin therapy by improving stability in the stomach, enhancing epithelial transport, and simplifying real-world use.4,5
Clinical outcomes
Across phase II data for the once-daily, non-peptide GLP-1R agonist orforglipron in obese adults without diabetes, mean weight loss of 9.4–14.7%6 was reported by week 36, depending on the dose, as compared to 2.3% with placebo.
Notably, 10% or more weight loss was observed in 46-75% of participants. Orforglipron treatment also improved several cardiometabolic measures, including waist circumference, systolic blood pressure, and fasting lipid levels.6
GI adverse events like nausea, vomiting, constipation, and diarrhea were the most common side effects. Nausea was often reported during dose escalation, with 10–17%6 of participants discontinuing treatment across dose cohorts due to GI symptoms; however, most events were mild to moderate and decreased after titration.
A daily oral GLP-1R agonist may overcome psychological and practical barriers to injections, thereby improving adherence for some patients. However, daily dosing requires consistent routines, whereas weekly injectables reduce dosing frequency. Injectable semaglutide (once weekly) remains more potent for weight loss than current oral peptide formulations, but oral agents may be preferable for individuals who avoid needles.
 Image Credit: algae / Shutterstock.com
Image Credit: algae / Shutterstock.com
The future of oral incretins
Emerging oral incretins signal a transition to scalable, needle-free therapies designed to sustain high efficacy while improving global access. Oral GLP-1 agents have been widely studied, with high-dose oral semaglutide currently under investigation for cardiovascular risk reduction but not yet established as reducing major adverse cardiovascular events.4 Orforglipron continues to demonstrate notable glycemic and weight effects in phase II trials.6
Multi-agonist strategies are increasingly being studied, some of which include dual GLP-1/GIP agonists like tirzepatide and triple agonists that target GLP-1, GIP, and glucagon-like retatrutide.7 Researchers have also investigated the potential utility of combining GLP-1 with sodium-glucose cotransporter-2 (SGLT-2) inhibitors, amylin analogs, or central appetite modulators. These combinations have been shown to enhance weight, glycemic, and cardiometabolic outcomes while potentially reducing GI side effects.7
The global rollout of these medications will be determined by their affordability and the efficiency of production processes. Today, the high prices and periodic shortages often observed with GLP-1R agonists limit equitable access, underscoring the importance of policy action, stable manufacturing, and fair distribution. Non-peptidic oral GLP-1RAs may eventually reduce supply pressures because they do not require peptide synthesis or cold-chain storage, but this will depend on scalable, cost-efficient manufacturing pathways.7
References
- Wojtara, M., Mazumder, A., Syeda, Y., & Mozgała, N. (2023). Glucagon‐like Peptide‐1 receptor agonists for chronic weight management. Advances in Medicine. DOI:10.1155/2023/9946924, https://www.hindawi.com/journals/amed/2023/9946924/
- Zheng, Z., Zong, Y., Ma, Y., et al. (2024). Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduction and Targeted Therapy 9(1). DOI:10.1038/s41392-024-01931-z, https://www.nature.com/articles/s41392-024-01931-z
- Zhu, Q., Chen, Z., Paul, P. K., et al. (2021). Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharmaceutica Sinica B 11(8); 2416-2448. DOI:10.1016/j.apsb.2021.04.001, https://www.sciencedirect.com/science/article/pii/S2211383521001167
- Rajput, R., Ghosh, S., Banerjee, S., et al. (2022). First-in-class oral semaglutide: Overcoming barriers of incretinisation in the Indian Context. Indian Journal of Endocrinology and Metabolism 26(5); 417-427. DOI:10.4103/ijem.ijem_217_22, https://journals.lww.com/indjem/fulltext/2022/09000/first_in_class_oral_semaglutide__overcoming.4.aspx
- Saldívar-Cerón, H. I., Vargas-Camacho, J. A., León-Cabrera, S., et al. (2025). Oral Small-Molecule GLP-1 Receptor Agonists: Mechanistic Insights and Emerging Therapeutic Strategies. Scientia Pharmaceutica 93(2). DOI:10.3390/scipharm93020026, https://www.mdpi.com/2218-0532/93/2/26
- Wharton, S., Blevins, T., Connery, L., et al. (2023). Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. New England Journal of Medicine 389(10); 877-888. DOI:10.1056/NEJMoa2302392, https://www.nejm.org/doi/full/10.1056/NEJMoa2302392
- Moiz, A., Filion, K. B., Tsoukas, M. A., et al. (2025). The expanding role of GLP-1 receptor agonists: a narrative review of current evidence and future directions. EClinicalMedicine 86. DOI:10.1016/j.eclinm.2025.103363, https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(25)00295-0/fulltext
Further Reading
Last Updated: Nov 18, 2025