Real-world data show that the GLP-1 receptor agonists semaglutide and tirzepatide not only manage blood sugar and weight but also mirror their heart-protective effects from clinical trials in everyday patients.

Study: Cardiovascular outcomes of semaglutide and tirzepatide for patients with type 2 diabetes in clinical practice. Image Credit: Love Employee / Shutterstock
In a recent study published in the journal Nature Medicine, researchers compared major adverse cardiovascular events (MACE) between glucagon-like peptide-1 (GLP-1) receptor agonists and comparators, benchmarking real-world estimates against randomized trial data to guide clinical decision-making.
Cardiovascular Risk and Therapeutic Relevance in Type 2 Diabetes
Adults with type 2 diabetes face an elevated lifetime risk of myocardial infarction and stroke, often transforming routine clinic visits into urgent cardiovascular events. New glucose-lowering drugs affect weight, blood pressure, and lipids, prompting questions about their cardiovascular impact in real-world care. While randomized controlled trials offer rigorous evidence, they may not fully represent older, multimorbid, or polytreated patients seen in primary care. This creates a need for evidence that reflects routine practice, while addressing the limitations of claims-based data, which may omit lifestyle variables such as diet, smoking, or exercise.
Study Framework and Data Sources
The investigators designed five active-comparator, new-user cohort studies using three major U.S. claims databases: Medicare Parts A, B, and D (2018–2020), Optum Clinformatics Data Mart (2018–February 2025), and Merative MarketScan (2018–2023). These datasets collectively capture de-identified longitudinal information on diagnoses, prescriptions, procedures, and healthcare utilization across diverse populations.
The study proceeded in three phases: emulation of the SUSTAIN-6 and SURPASS-CVOT trials, expansion to real-world cohorts, and a direct comparison between tirzepatide and semaglutide. Participants included adults with type 2 diabetes and obesity, stratified by cardiovascular risk, including established cardiovascular disease, NYHA class II–III heart failure, or chronic kidney disease.
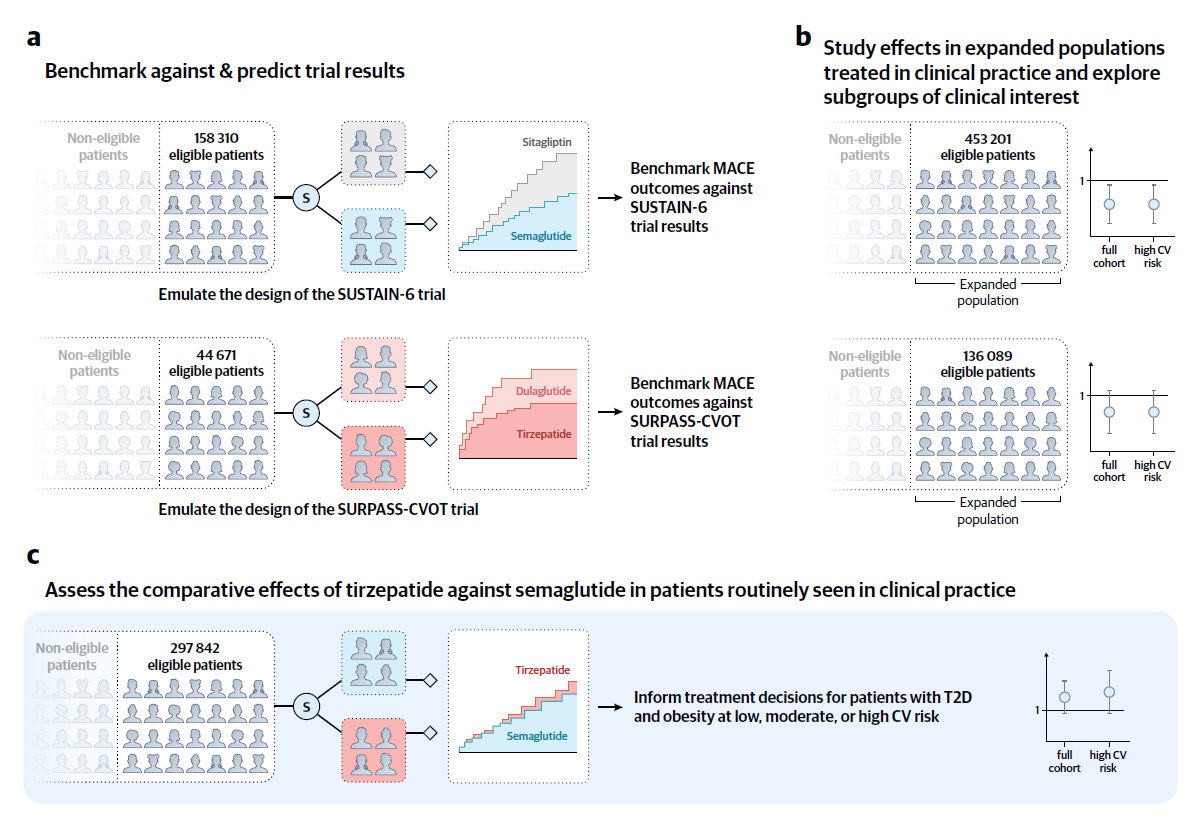
Overview of the study design to assess the comparative effects of semaglutide and tirzepatide in patients at cardiovascular risk. The study proceeded in three sequential steps. (a) We emulated the design of the SUSTAIN-6 and SURPASS-CVOT trials using three United States claims databases to benchmark the trial emulations against the reference trials and predict results. (b) We expanded the patient populations within this framework to assess the effectiveness of each agent in clinical practice. (c) We compared tirzepatide versus semaglutide in a head-to-head comparison to inform clinical decision-making. Abbreviations: CV = cardiovascular; MACE = major adverse cardiovascular events; S = selection of patients initiating semaglutide, tirzepatide, dulaglutide, or sitagliptin (as a placebo proxy) via propensity score 1:1 nearest neighbor matching to mimic randomization; T2D = type 2 diabetes.
Endpoint Definitions and Statistical Approach
The primary endpoint was MACE, defined as all-cause mortality, myocardial infarction, or stroke, assessed over 52 weeks using an as-treated design. Propensity-score matching (1:1, caliper 0.01) was used to balance the treatment groups, and risk estimates were generated using Kaplan–Meier curves, Cox proportional hazards models, and fixed-effects meta-analysis. Concordance with randomized trials was assessed using RCT-DUPLICATE predefined benchmarks to evaluate consistency in both direction and magnitude.
Validation Against Randomized Clinical Trials
Benchmarking analyses demonstrated close agreement with reference trials. For the SURPASS-CVOT emulation (tirzepatide vs dulaglutide), the hazard ratio for MACE was 0.83 (95% CI, 0.69–1.01) compared with 0.92 (95% CI, 0.83–1.01) in the trial, satisfying all concordance criteria. Similarly, the SUSTAIN-6 emulation (semaglutide vs sitagliptin) reproduced trial-aligned estimates, except for all-cause mortality, which showed potential residual confounding and led to later methodological refinements that emphasized myocardial infarction and stroke endpoints.
Real-World Effectiveness Across Drug Comparisons
In expanded routine-care populations, semaglutide reduced the 1-year risk of myocardial infarction or stroke to 1.5% compared with 1.7% with sitagliptin (HR, 0.82; 95% CI, 0.74–0.91). For tirzepatide versus dulaglutide, MACE risk was 1.4% versus 1.8% (HR 0.87; 95% CI, 0.75–1.01), consistent across risk groups. In the head-to-head tirzepatide versus semaglutide comparison, both agents demonstrated equivalent cardiovascular outcomes (HR 1.06; 95% CI, 0.95–1.18).
Secondary outcomes showed semaglutide reduced myocardial infarction (HR 0.81) and stroke (HR 0.84) versus sitagliptin, while tirzepatide was non-inferior to dulaglutide for mortality, myocardial infarction, and stroke. Composite heart-failure outcomes favored both GLP-1 agents, with semaglutide and tirzepatide each reducing hospitalization or urgent visits.
Subgroup and Sensitivity Findings
Analyses stratified by age, sex, SGLT2 inhibitor co-use, and cardiovascular risk revealed no significant heterogeneity, supporting the external validity of the findings across typical clinical populations. A numerically lower MACE was observed with semaglutide, while tirzepatide demonstrated favorable heart failure profiles. However, limitations of claims data, particularly regarding behavioral and clinical details, necessitate cautious interpretation of causal inferences.
Implications for Clinical Practice and Policy
In real-world care, semaglutide lowered MACE compared with sitagliptin, and tirzepatide achieved cardiovascular effectiveness comparable to dulaglutide and semaglutide. These results aligned with randomized trial evidence, reinforcing confidence that observed benefits extend beyond controlled settings. For patients with type 2 diabetes, GLP-1 receptor agonists not only support glycemic and weight management but also confer meaningful cardiovascular protection. For health systems, these findings endorse the broader application of GLP-1 therapies to mitigate cardiovascular risk in everyday practice.
Journal reference:
- Krüger, N., Schneeweiss, S., Desai, R. J., Kattinakere Sreedhara, S., Kehoe, A. R., Fuse, K., Hahn, G., Schunkert, H., & Wang, S. V. (2025). Cardiovascular outcomes of semaglutide and tirzepatide for patients with type 2 diabetes in clinical practice. Nature Medicine. DOI: 10.1038/s41591-025-04102-x