2. What should I know before I receive Prolia?
Do not use if you have ever had an allergic reaction to denosumab, medicines produced
using Chinese Hamster Ovary cells or any of the ingredients listed at the end of the
CMI.
Do not use in patients under 18 years of age.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
Tell your doctor if you have calcium deficiency.
3. What if I am taking other medicines?
Some medicines may interfere with Prolia and affect how it works.
4. How do I use Prolia?
The recommended dose of Prolia is 60 mg given once every 6 months as a single injection
under the skin.
5. What should I know while using Prolia?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using Prolia.
Take calcium and vitamin D supplements if your doctor has told you to.
Maintain good oral hygiene when being treated with Prolia.
Attend all of your treatment and doctor's appointments so that your progress can be
checked.
Tell your doctor immediately if you become pregnant while taking Prolia.
|
|
Things you should not do
|
Do not stop or delay Prolia treatment without talking to your doctor.
|
|
Looking after your medicine
|
Store Prolia in the refrigerator (2°C to 8°C) in the original pack to protect from
light. Do not freeze.
Do not shake or vigorously agitate the pre-filled syringe.
|
6. Are there any side effects?
Side effects that require urgent medical attention include: signs of an allergic reaction;
muscle aches, twitches or cramps; numbness or tingling in your fingers, toes or around
your mouth; persistent pain or swelling and/or non-healing sores in your mouth or
jaw; pain in your hip, groin, or thigh, which is sometimes severe; severe allergic
reaction with skin rash, blisters or fever.
Active ingredient(s):
denosumab
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using Prolia. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using Prolia.
Where to find information in this leaflet:
1. Why am I using Prolia?
Prolia contains the active ingredient denosumab. Denosumab is a protein (monoclonal antibody) that attaches (binds) specifically to
another unique protein in the body in order to stop the development of bone-removing
cells before they reach the bones and cause damage. Continued treatment with Prolia
makes your bone stronger and less likely to break.
Prolia is used to:
Treat osteoporosis in women after the menopause, to reduce the risk of spinal, non-spinal
and hip fractures.
Treat bone loss in men with osteoporosis at increased risk of fracture.
Treat bone loss that results from a reduction in testosterone level caused by surgery
or treatment with drugs in men with prostate cancer.
Improve bone density in patients treated with corticosteroids.
Bone is a living tissue and is renewed all the time. In women, the ovaries produce
the hormone oestrogen which helps keep bones healthy. After menopause, the oestrogen
level drops which affects the bone renewal cycle so that more bone is lost than made,
resulting in a lower bone mass. This leaves bones thin and fragile. Osteoporosis is
the term used to describe an increased fracture risk, usually with low bone density.
Osteoporosis becomes more common with increasing age. It is more common in women.
It can also occur in patients receiving corticosteroids. Many people with osteoporosis
have no symptoms but they are still at risk of breaking bones (developing fractures),
especially in the spine, hips and wrists.
Other things that can increase the risk of fractures include:
age
existence of a previous fracture
family history of hip fractures
low body weight
drinking alcohol
smoking.
Prolia is prescribed to improve your bone density and to reduce your risk of fracture.
Surgery or medicines used in the treatment of men with prostate cancer to stop the
production of testosterone can also lead to bone loss. The bones become weaker and
break more easily.
Your doctor, however, may have prescribed Prolia for another reason.
2. What should I know before I use Prolia?
Warnings
Do not use Prolia if:
you have low calcium levels in your blood (hypocalcaemia). Your doctor may do a blood
test to check your calcium levels before you use Prolia.
you are pregnant, think you may be pregnant, or trying to get pregnant. Prolia may
harm your unborn baby.
you are breast-feeding. It is not known if the active ingredient, denosumab, passes
into breast milk.
you are allergic to denosumab, any medicines that are produced using Chinese Hamster
Ovary cells, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
you are a child or adolescent. Prolia is not indicated for use in patients under 18
years of age.
you are taking another medicine containing denosumab.
the expiry date [EXP.] printed on the pack has passed. If you use it after the expiry
date has passed, it may not work as well.
the packaging is torn or shows signs of tampering.
the Prolia solution is cloudy or discoloured. There may be some translucent to white
particles of protein in the solution, however the medicine can still be used.
Check with your doctor if you:
have allergies to any other medicines, or any other substances such as foods, preservatives
or dyes.
have calcium or vitamin D deficiency. If you are prone to low calcium levels, your
doctor will monitor your blood especially in the first few weeks after starting Prolia.
Severe low blood calcium levels may lead to hospitalisation, life-threatening events
and death.
are unable to take daily calcium or vitamin D supplements.
have or have had severe kidney problems, kidney failure or have needed dialysis, which
may increase your chance of getting low blood calcium if you do not take calcium supplements.
had or have pain in the teeth, gums or jaw, swelling or numbness of the jaw, a "heavy
jaw feeling" or loosening of a tooth. A dental condition called jaw osteonecrosis
has been rarely reported in patients treated with Prolia. Your doctor should examine
your mouth and may ask for a dental examination before you start Prolia. You may need
to have dental treatment completed before starting your medicine.
You may have higher risk of developing jaw problems if you:
- are undergoing chemotherapy, taking steroids, or are having a dental procedure
- do not receive routine dental care, have gum disease or have taken Prolia for a
long time
have been told by a doctor or other healthcare professional that you have an intolerance
to some sugars.
take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy
Prolia has not been tested in pregnant women. Do not use Prolia if you are pregnant,
think you may be pregnant, or trying to get pregnant. Prolia may harm your unborn
baby.
Breastfeeding
Do not use Prolia if you are breast-feeding. It is not known if the active ingredient,
denosumab, passes into breast milk. It is important to talk to your doctor if you
are breast-feeding or plan to do so. Your doctor will then help you decide whether
to stop breast-feeding or whether to stop taking Prolia.
Use in children
Do not use Prolia in children or adolescents under 18 years of age.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Tell your doctor if you are taking another medicine containing denosumab (Xgeva).
If you are taking Xgeva, and/or other medicines containing denosumab, you should not
take Prolia.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect Prolia.
4. How will I be given Prolia?
How is Prolia given
Prolia is given as an injection under the skin. This is called a subcutaneous injection.
How much Prolia will I be given
For each dose of Prolia you will be given 60 mg as a single injection.
When you will be given Prolia
Prolia is injected once every 6 months.
It is important to take your dose of Prolia on schedule. Do not skip or delay getting
your injection.
Each pack of Prolia contains a reminder card that can be removed from the carton.
Use the reminder card to keep a record of the next injection date.
Continue using Prolia for as long as your doctor tells you to. Prolia can treat osteoporosis
and bone loss only for as long as you keep having treatment. Please talk to your doctor
before you consider stopping treatment.
You should also take calcium and vitamin D supplements while receiving Prolia. Prolia
may lower the calcium levels in your blood. Your doctor, nurse or pharmacist will
discuss how much calcium and vitamin D you should take to help prevent low calcium
levels.
How long will I be given Prolia
Continue receiving your Prolia injections for as long as your doctor tells you to.
Prolia helps control your condition but does not cure it. Therefore, you must be given
Prolia treatment every 6 months.
Do not stop or delay Prolia treatment, without checking with your doctor.
Stopping, skipping or delaying Prolia treatment may worsen your condition or increase
your chance of breaking bones, including bones in your spine, especially if you have
a history of broken bones in your spine.
If your Prolia treatment is stopped or delayed, discuss other available treatment
options with your doctor.
Instructions for injecting Prolia when supplied in a pre-filled syringe with an automatic
needle guard
This section contains information on how you, or the person injecting you (your carer),
must use the Prolia pre-filled syringe.
To reduce the risk of accidental injury by the needle, each pre-filled syringe is
equipped with an automatic needle guard that is automatically activated to cover the
needle after complete delivery of the pre-filled syringe contents.
Guide to parts
Before and after use
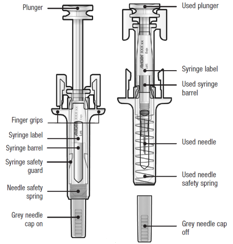
Important
Before you or your carer use Prolia pre-filled syringe with automatic needle guard,
read this important information:
It is important that you or your carer do not try to give the injection unless training
from a doctor, nurse or pharmacist has been received.
Prolia is given as an injection into the tissue just under the skin (subcutaneous
injection).
DO NOT remove the grey needle cap from the pre-filled syringe until you are ready
to inject.
DO NOT use the pre-filled syringe if it has been dropped on a hard surface. Use a
new pre-filled syringe and call your doctor, nurse or pharmacist.
DO NOT attempt to activate the pre-filled syringe prior to injection.
DO NOT attempt to remove the clear pre-filled syringe safety guard from the pre-filled
syringe.
Call your doctor, nurse or pharmacist if you have any questions.
Step 1: Prepare
A: Remove the pre-filled syringe tray from the package and gather the supplies needed
for your injection: alcohol wipes, a cotton ball or gauze pad, a plaster and a sharps
disposal container (not included).
For a more comfortable injection, leave the pre-filled syringe at room temperature
for about 30 minutes before injecting. Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the new pre-filled syringe and the other
supplies.
DO NOT try to warm the syringe by using a heat source such as hot water or microwave.
DO NOT leave the pre-filled syringe exposed to direct sunlight.
DO NOT shake the pre-filled syringe.
Keep pre-filled syringes out of the sight and reach of children.
B: Open the tray, peeling away the cover. Grab the pre-filled syringe safety guard
to remove the pre-filled syringe from the tray.
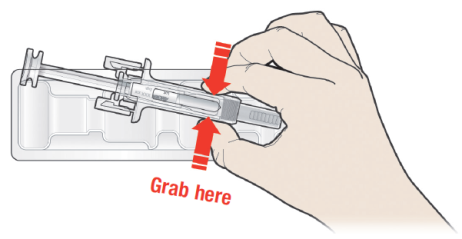
For safety reasons:
DO NOT grasp the plunger.
DO NOT grasp the grey needle cap.
C: Inspect the medicine and pre-filled syringe
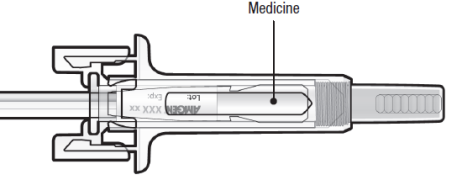
DO NOT use the pre-filled syringe if:
The medicine is cloudy or there are particles in it. It must be a clear, colourless
to slightly yellow solution.
Any part appears cracked or broken.
The grey needle cap is missing or not securely attached.
The expiry date printed on the label has passed the last day of the month shown.
In all cases, call your doctor, nurse or pharmacist.
Step 2: Get ready
A: Wash your hands thoroughly. Prepare and clean your injection site.
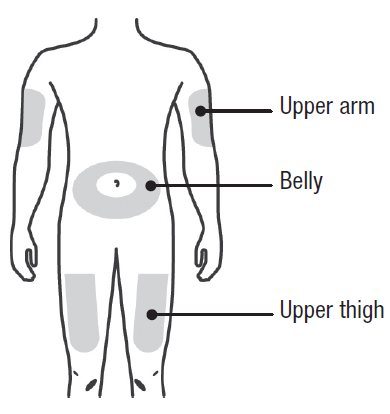
You can use:
Upper part of your thigh.
Belly, except for a 5 cm (2-inch) area right around your belly button.
Outer area of upper arm (only if someone else is giving you the injection).
Clean the injection site with an alcohol wipe. Let the skin dry.
DO NOT touch the injection site before injecting.
DO NOT inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting
into areas with scars or stretch marks.
B: Carefully pull the grey needle cap straight out and away from your body.
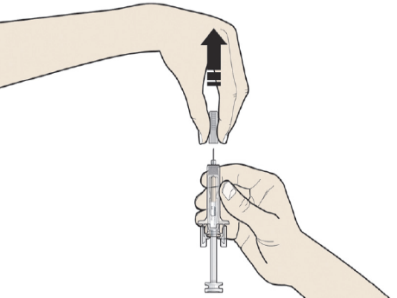
C: Pinch the injection site to create a firm surface.
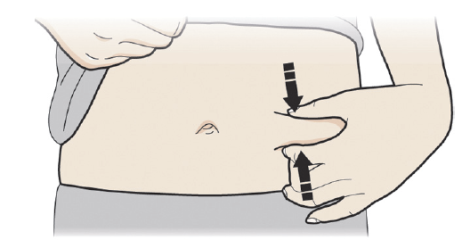
It is important to keep the skin pinched when injecting.
Step 3: Inject
A: Hold the pinch. INSERT the needle into skin.
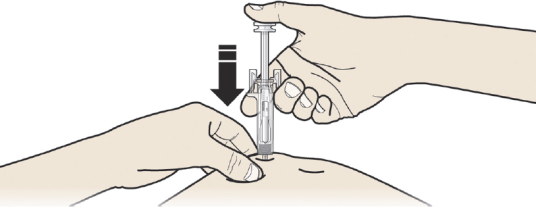
DO NOT touch the cleaned area of the skin.
B: PUSH the plunger with slow and constant pressure until you feel or hear a "snap".
Push all the way down through the snap.
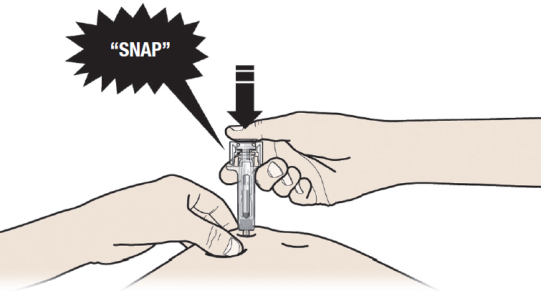
It is important to push down through the "snap" to deliver your full dose.
C: RELEASE your thumb. Then LIFT the syringe off the skin.
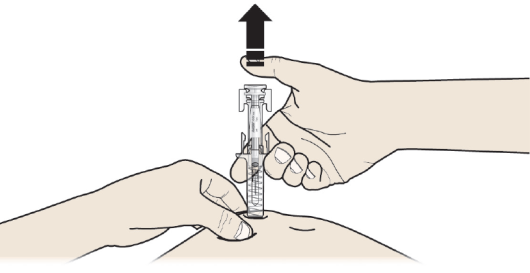
After releasing the plunger, the pre-filled syringe safety guard will safely cover
the injection needle.
DO NOT put the grey needle cap back on used pre-filled syringes.
Step 4: Finish
A: Discard the used pre-filled syringe and other supplies
in a sharps disposal container.
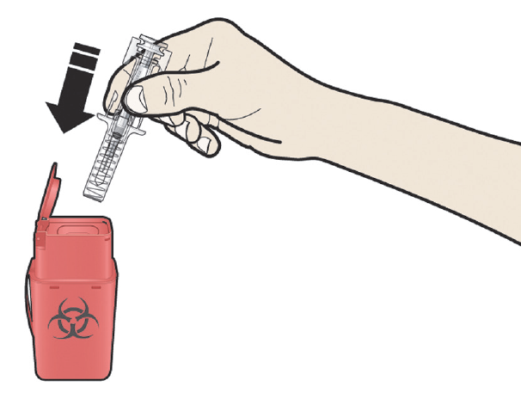
Medicines should be disposed of in accordance with local requirements. Ask your pharmacist
how to dispose of medicines no longer required. These measures will help to protect
the environment.
Keep the syringe and sharps disposal container out of sight and reach of children.
DO NOT reuse the pre-filled syringe.
DO NOT recycle pre-filled syringes or throw them into household waste.
B: Examine the injection site
If there is blood, press a cotton ball or gauze pad on your injection site. DO NOT
rub the injection site. Apply a plaster if needed.
If you miss a dose of Prolia
If you miss a dose, Prolia should be administered as soon as possible. From then on,
Prolia should be scheduled every 6 months from the date of the last injection.
If you are given too much Prolia
If you think you or anyone else has received too much Prolia, you should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Prolia?
Things you should do
take calcium and vitamin D supplements if your doctor has told you to. Most people
do not get enough calcium and vitamin D in their diet and supplements are needed to
help strengthen bones.
practice good dental hygiene while being treated with Prolia.
Your routine dental hygiene should include brushing your teeth and tongue after every
meal, including the evening and gentle flossing once a day to remove plaque.
Use a mirror and check your teeth and gums regularly for any changes such as sores
or bleeding gums. If you notice any problems, tell your doctor and dentist immediately.
tell any doctor, dentist, nurse, or pharmacist you visit that you are using Prolia.
if you are about to be started on any other medicine, remind your doctor, nurse or
pharmacist that you are being treated with Prolia.
attend all your doctor's appointments so that your progress can be checked. Your doctor
may recommend you to have some tests, X-rays and/or bone density scans from time to
time to make sure the medicine is working.
Call your doctor straight away if you:
have spasms, twitches, aches or cramps in your muscles, and/or numbness or tingling
in your fingers, toes or around your mouth, or have seizures. You may have low levels
of calcium in your blood.
experience any problems with your mouth or teeth such as loose teeth or ill-fitting
dentures, pain, or swelling while being treated with Prolia.
develop a swollen, red area of skin, most commonly in the lower leg, that feels hot
and tender (cellulitis) and sometimes experienced with fever and chills.
experience new or unusual pain in your hip, groin, or thigh. Some people have developed
unusual fractures in their thigh bone while being treated with Prolia. These fractures
may occur with little or no trauma and may involve both thigh bones. This side effect
is very rare.
experience shortness of breath; wheezing or difficulty breathing; swelling of the
face, lips, tongue, throat or other parts of the body; rash, itching or hives on the
skin. If you experience any of these side effects you may be having an allergic reaction
to Prolia. These side effects are rare.
have a severe allergic reaction with skin rash, blisters or fever
notice any purple or brownish-red spots, hives or skin sores. This may be an allergic
reaction that can damage blood vessels mainly in the skin. This side effect is very
rare.
become pregnant while using Prolia. Your doctor can discuss with you the risks of
having it while you are pregnant.
Females that are menstruating should ensure they have adequate contraception while
taking Prolia.
Things you should not do
Do not stop using Prolia without talking to your doctor. After your treatment with
Prolia is stopped, or if you skip or delay taking a dose, your risk of breaking bones
in your spine is increased, especially if you have a history of broken bones in the
spine. If your Prolia treatment is stopped, discuss other available treatment options
with your doctor.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Prolia
affects you.
Prolia has no known effects on the ability to drive or use machines but, as a general
precaution, if you are driving soon after an injection, arrange to have someone else
drive.
Looking after your medicine
If you need to store your Prolia before use, follow the instructions in the carton
on how to take care of your medicine properly.
Store Prolia in the refrigerator between 2 and 8°C. Do not freeze.
Keep your medicine in the original carton to protect from light. If you remove the
medicine from the carton it may not keep well.
Your medicine may be left outside the refrigerator to reach room temperature (up to
25°C) before injection. This will make the injection more comfortable.
Once your medicine has been left to reach room temperature (up to 25°C), it must be
used within 30 days.
Do not keep Prolia at temperatures above 25°C. Warm temperatures will affect how Prolia
works.
Do not shake or vigorously agitate the pre-filled syringe.
Keep it where young children cannot reach it.
When to discard your medicine
Prolia is for single-use in one patient only. Dispose of any unused or expired medicine
as instructed below.
Getting rid of any unwanted medicine
Your doctor or nurse is likely to dispose of Prolia for you. However, if you need
to get rid of this medicine because you no longer need to use it or it is out of date,
take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Prolia contains
|
Active ingredient
(main ingredient)
|
denosumab
|
|
Other ingredients
(inactive ingredients)
|
Acetate
sodium hydroxide
sorbitol
polysorbate 20
water for injections
|
Do not take this medicine if you are allergic to any of these ingredients.
What Prolia looks like
Prolia is a clear, colourless to slightly yellow solution supplied in a pre-filled
syringe with an automatic needle guard.
Prolia comes in a single pack size containing 60 mg of denosumab in a volume of 1.0
mL (60 mg/1.0 mL). Aust R 159323.
Each pack contains one ready to use single-use pre-filled syringe and one reminder
card.
The needle cover on the pre-filled syringe is not made with natural rubber latex.
Who distributes Prolia
Amgen Australia Pty Ltd
Level 11, 10 Carrington St
Sydney NSW 2000
Ph: 1800 803 638
This leaflet was prepared in October 2025.