Introduction
Defining cancer plasticity
How cancer cells adapt?
Clinical implications
Recent advances and research
Looking ahead: Therapeutic strategies and hope
Conclusions
References
Further reading
From shape-shifting cells to immune escape, cancer’s plasticity thwarts even the best therapies, but scientists are closing in with smarter, adaptive treatments that could rewrite the future of oncology.
 Image Credit: Lightspring / Shutterstock.com
Image Credit: Lightspring / Shutterstock.com
Introduction
Cancer plasticity refers to the remarkable ability of cancer cells to alter their identity, function, and behavior in response to environmental stressors or therapeutic pressures. This dynamic trait allows cancer cells to transition between different phenotypes, thereby contributing to tumor heterogeneity, disease progression, and resistance to treatment.
Through mechanisms like epithelial-mesenchymal transition (EMT) and the acquisition of stem-like properties, cancer plasticity enables tumors to adapt and survive, even after exposure to targeted therapies. Understanding and targeting this adaptability is critical for developing more effective treatments and overcoming the persistent challenge of drug resistance in cancer care.1
Defining cancer plasticity
Cancer plasticity refers to the ability of cancer cells to reversibly alter their phenotype in response to internal factors, such as epigenetic modifications, or external signals from the tumor microenvironment (TME). Unlike genetic mutations, which are permanent changes in the deoxyribonucleic acid (DNA) sequence, plasticity involves non-genetic and reversible shifts that enable cancer cells to survive treatment, spread to other tissues, and evade cell death.1,2
The EMT is critically involved in cancer plasticity, as this process allows epithelial cells to acquire mesenchymal properties, including increased mobility and invasiveness. EMT is regulated by transcription factors such as zinc finger protein SNAI1 (SNAIL), zinc finger E-box-binding homeobox (ZEB), and Twist-related protein 1 (TWIST).
Stemness and transdifferentiation are different forms of plasticity that contribute to cancer progression, therapy resistance, and metastasis.
Transdifferentiation, which is defined as the direct conversion of one mature cell type into another without returning to a pluripotent state, allows cancer cells to adopt functions like vascular mimicry or immune evasion.1,2 Comparatively, stemness refers to the acquisition of stem cell-like traits, such as the capacity to self-renew and differentiate into multiple cell types. This supports tumor heterogeneity and increases the risk of relapse.
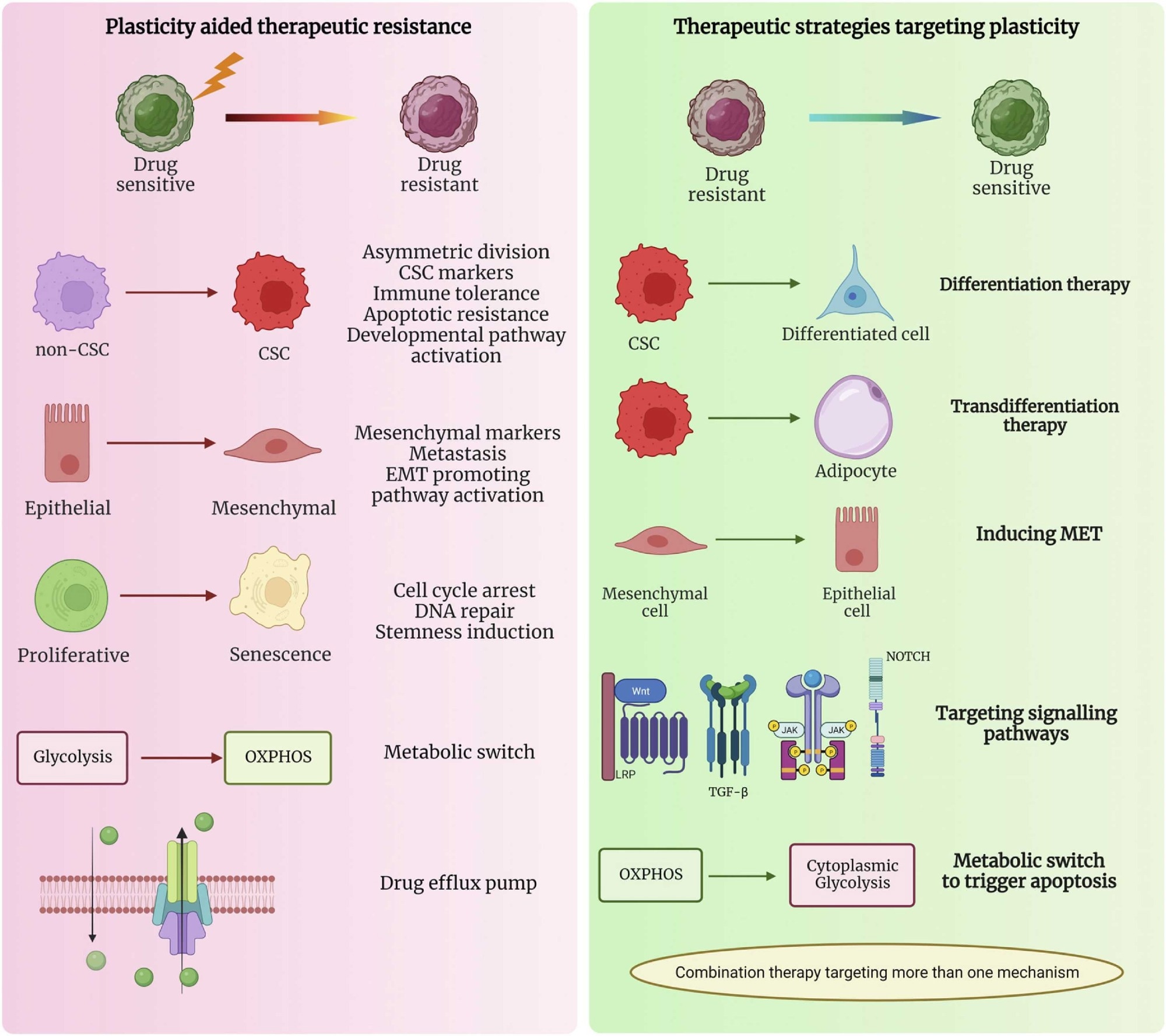 Plasticity-assisted mechanisms involved in the switch from drug sensitive to drug resistant phenotype and the therapeutic strategies targeting plasticity that can be used in cancer treatment (created using BioRender).2
Plasticity-assisted mechanisms involved in the switch from drug sensitive to drug resistant phenotype and the therapeutic strategies targeting plasticity that can be used in cancer treatment (created using BioRender).2
How cancer cells adapt?
Cancer cells exhibit remarkable adaptability, which enables their survival under therapeutic pressure and their ability to evade immune defenses. One major adaptive behavior is therapy resistance, which is often driven by reversible epigenetic changes, such as modifications in DNA methylation or histone structure, that alter gene expression without permanently changing the genetic code.
Another critical mechanism is lineage plasticity. For example, in prostate and lung cancers, tumor cells can change their identity from hormone receptor-dependent to neuroendocrine-like phenotypes, thereby reducing the efficacy of targeted therapies.3
Tumor cells have also evolved various mechanisms to evade the host immune system. For example, some cancer cells can increase the expression of programmed death-ligand 1 (PD-L1), which interacts with programmed cell death protein 1 (PD-1) on T-cells to suppress the immune response.
Cancer cells can modify cytokine release to attract immunosuppressive immune cells. Metabolic shifts also support survival, as cancer cells often switch from oxidative phosphorylation to glycolysis or increase fatty acid metabolism to meet energy demands.3
These adaptations promote tumor heterogeneity and intratumoral evolution, leading to the development of diverse subpopulations within a tumor. This tumor microenvironment, which includes immune cells, fibroblasts, and the extracellular matrix, supports this plasticity to promote disease progression.3
Clinical implications
Cancer cell plasticity creates major challenges in diagnosing, predicting, and treating cancer. Because cancer cells can shift their identity in response to environmental stressors or therapy, biomarkers may change over time, which increases the complexity of accurately classifying tumors and predicting their response to different treatments.
This unpredictability limits the effectiveness of precision medicine, which relies on stable molecular targets. For example, lung cancer cells can transition between adenocarcinoma and squamous cell types or adopt stem-like characteristics. These heterogeneities have the potential to prevent clinicians from accurately determining whether a lesion is premalignant, aggressive, or likely to respond to a specific therapy.4
Cancer cells can also reprogram their signaling pathways or change how they interact with the immune system to evade destruction. Such adaptive changes enable their survival, which subsequently increases the risk of treatment failure, disease recurrence, and metastasis.
Understanding the factors that promote these transitions, including influences from the tumor microenvironment, is essential to developing more durable and adaptable treatment strategies.4
How and why does cancer treatment resistance develop?
Recent advances and research
Innovative tools, such as lineage tracing, single-cell ribonucleic acid sequencing (scRNA-seq), and three-dimensional (3D) tumor models, have enabled the detailed mapping of tumor heterogeneity and plasticity. These technologies allow researchers to explore how cancer cells dynamically shift between different phenotypic states, evade treatment, and adapt to changing microenvironments.5,6
Promising strategies have emerged to target EMT and cancer stem cells (CSCs), two key mechanisms driving plasticity. For example, in gastric adenocarcinoma, the transcription factor SRY-box transcription factor 9 (SOX9) was found to regulate stemness. By targeting the leukemia inhibitory factor/leukemia inhibitory factor receptor (LIF/LIFR) signaling pathway, researchers effectively impaired CSC survival.
Novel therapies aim to pharmacologically “lock” cancer cells into less aggressive or drug-sensitive states. The small-molecule inhibitor JQ1, which targets the bromodomain-containing protein 4 (BRD4), can reverse resistance traits associated with histone acetylation. Targeting wingless-related integration site (Wnt) or Janus kinase/signal transducer and activator of transcription (Jak-STAT) pathways, both of which are implicated in phenotypic switching and immune evasion, has also been widely studied.5,6
Collectively, these advances reflect a growing shift in cancer treatment strategies, from solely targeting genetic mutations to addressing the dynamic and reversible changes in cancer cell identity. This evolving focus holds promise for improving long-term outcomes and overcoming therapy resistance.5,6
Looking ahead: Therapeutic strategies and hope
The evolving landscape of cancer therapy is increasingly shaped by breakthroughs targeting epigenetic plasticity and tumor heterogeneity. Emerging treatments include epigenetic drugs, plasticity inhibitors, and combination regimens that integrate DNA methyltransferase inhibitors, histone modification modulators, and non-coding RNA-based therapies.
These approaches aim to reverse abnormal gene expression patterns that contribute to tumor progression and resistance. For example, therapies targeting lysine demethylase 4 (KDM4) and DNA methyltransferase enzymes have shown promise in reprogramming cancer cells into more treatment-responsive states.7
One of the most hopeful advances is the combination of epigenetic therapies with immune checkpoint inhibitors, which re-sensitize tumors to immune detection. Clinical trials involving microRNA mimics and histone deacetylase inhibitors have also reported some success in specific cancer types, particularly hematological malignancies and triple-negative breast cancer (TNBC). These strategies are reinforced by innovations in personalized medicine, where patient-specific epigenetic profiles guide therapeutic decisions.7
Despite the challenges of tumor plasticity and resistance, continued funding and research into the dynamic cancer epigenome are paving the way for individualized and adaptive therapies. With every clinical trial and preclinical innovation, the vision of a future where cancer care is both more effective and precisely tailored comes closer to reality, offering renewed hope for long-term remission and improved survival.7
Conclusions
As cancer cells adapt by changing their identity and function, they evade conventional therapies and challenge precision oncology. Recognizing this dynamic behavior offers new opportunities to develop more effective and personalized treatment strategies.
However, advancing these approaches requires sustained support for scientific research, patient advocacy, and investment in adaptive cancer care. By prioritizing innovation and collaboration, the medical community can better respond to the evolving nature of cancer, ultimately improving survival outcomes.
References
- Bhat, G.R., Sethi, I., Sadida, H.Q., Rah, B., Mir, R., Algehainy, N., Albalawi, I.A., Masoodi, T., Subbaraj, G.K., Jamal, F. and Singh, M. (2024). Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer and Metastasis Reviews, 43(1), 197-228. DOI: 10.1007/s10555-024-10172-z, https://link.springer.com/article/10.1007/s10555-024-10172-z
- Warrier, N. M., Kelkar, N., Johnson, C. T., Govindarajan, T., Prabhu, V., & Kumar, P. (2023). Understanding cancer stem cells and plasticity: Towards better therapeutics. European Journal of Cell Biology, 102(2), 151321. DOI:10.1016/j.ejcb.2023.151321, https://www.sciencedirect.com/science/article/pii/S0171933523000365
- De Visser, K. E., & Joyce, J. A. (2023). The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer cell, 41(3), 374-403.DOI:10.1016/j.ccell.2023.02.016, https://www.sciencedirect.com/science/article/pii/S1535610823000442
- Moghaddam, S.J., Savai, R., Salehi-Rad, R., Sengupta, S., Kammer, M.N., Massion, P., Beane, J.E., Ostrin, E.J., Priolo, C., Tennis, M.A. and Stabile, L.P. (2024). Premalignant progression in the lung: knowledge gaps and novel opportunities for interception of non–small cell lung cancer. An Official American Thoracic Society Research Statement. American Journal of Respiratory and Critical Care Medicine, 210(5), 548-571. DOI:10.1164/rccm.202406-1168ST, https://www.atsjournals.org/doi/10.1164/rccm.202406-1168ST
- Qin, S., Jiang, J., Lu, Y., Nice, E. C., Huang, C., Zhang, J., & He, W. (2020). Emerging role of tumor cell plasticity in modifying therapeutic response. Signal transduction and targeted therapy, 5(1), 228. DOI:10.1038/s41392-020-00313-5, https://www.nature.com/articles/s41392-020-00313-5
- Liu, B., Hu, S., & Wang, X. (2024). Applications of single-cell technologies in drug discovery for tumor treatment. Iscience. DOI:10.1016/j.isci.2024.110486, https://www.sciencedirect.com/science/article/pii/S2589004224017115
- Sadida, H. Q., Abdulla, A., Al Marzooqi, S., Hashem, S., Macha, M. A., Akil, A. S. A. S., & Bhat, A. A. (2024). Epigenetic modifications: Key players in cancer heterogeneity and drug resistance. Translational oncology, 39, 101821. DOI:10.1016/j.tranon.2023.101821, https://www.sciencedirect.com/science/article/pii/S1936523323002073
Further Reading
Last Updated: Jun 16, 2025