This article and associated images are based on a poster originally authored by Amy Harmens, Cansu Karyal, Han Yin, Elisha Moran, Annabelle Herrington-Symes, Vincentius Aji Jatikusumo and Beth Wensley and presented at ELRIG Drug Discovery 2025 in affiliation with LifeArc.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Summary
Our mission was to humanize an antibody to enter clinical trials as a prophylactic and therapeutic treatment against CCFHV1.
We aimed to produce 1 mg of GP38 antigen at > 95 % purity for five strains of the virus.
Initial trials showed very low yield, purity, and poor QC results.
Optimized methods now yield 8-15 mg/L at 100 % purity for all required strains.
Crimean-Congo Haemorrhagic Fever Virus (CCHFV)
- CCHFV is a tick-borne virus that causes severe haemorrhagic fever, which is fatal in over 30 % of cases2.
- The WHO recognizes CCHFV as a priority pathogen due to its high fatality rate and lack of approved treatments.
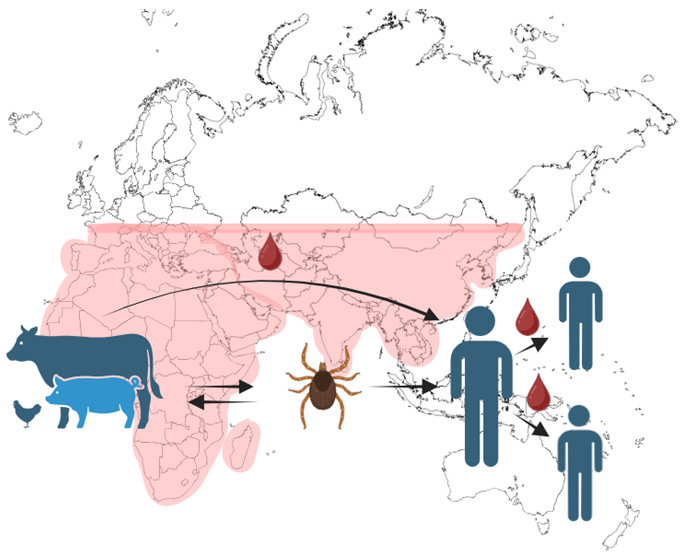
No approved vaccine or therapeutic. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
GP38 viral antigen
CCHFV M segment:
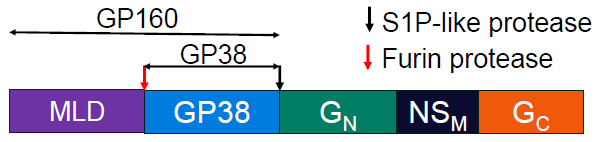
Figure 1. The M-segment encodes a glycoprotein precursor which is cleaved to form several mature glycoproteins3. MLD may be required for the correct folding and secretion of GP38. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Project requirements:
- 1 mg of GP38 at > 95 % purity
- 5 strains of CCHFV (A-E; 70-95 % AA sequence identity)
Initial results
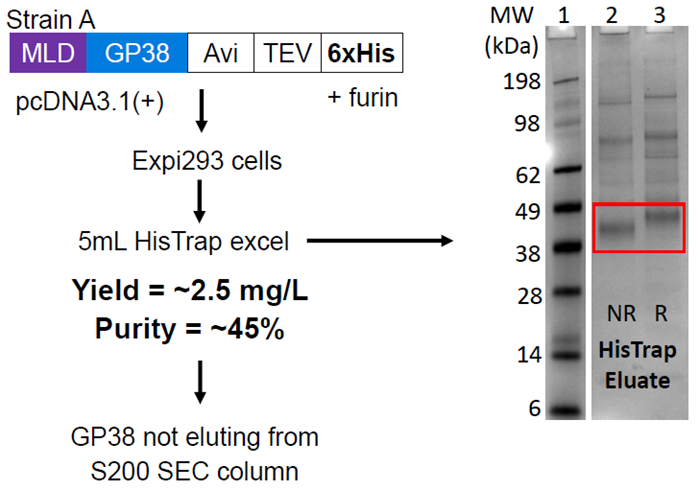
Figure 2. SDS-PAGE gel of HisTrap eluate shows GP38 with many host cell contaminants. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Optimized methods
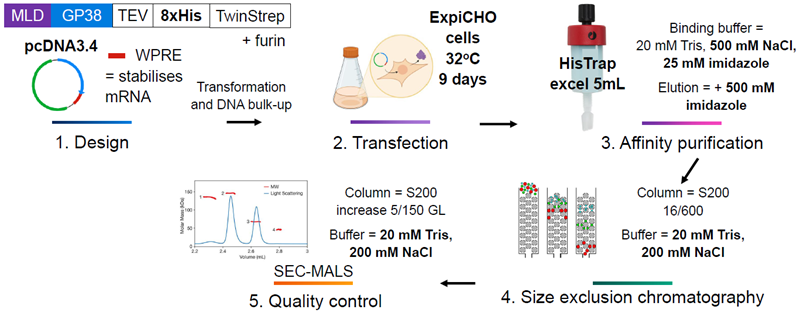
Figure 3. Final methods used to produce all strains of GP38 following optimization. Created with BioRender.com. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Results
8-fold increase in expression yield achieved through parallel optimization of construct design and expression conditions
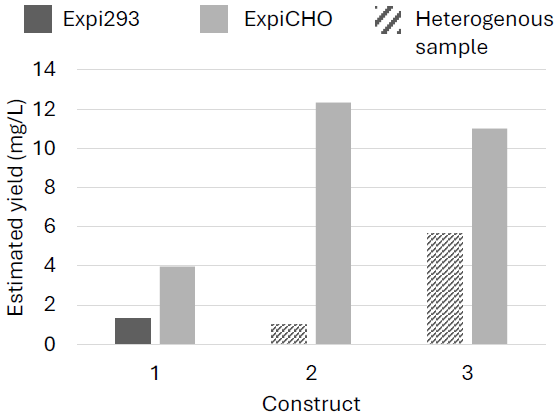
| Construct |
Tags |
Vector |
| 1 |
Avi_TEV_6xHis |
pcDNA3.1(+) |
| 2 |
TEV_8xHis_TwinStrep |
pcDNA3.1(+) |
| 3 |
TEV_8xHis_TwinStrep |
pcDNA3.4 |
Figure 4. Estimated expression yields of GP38 strain A based on the concentration of NiNTA magnetic beads eluate. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
100 % purity and good profile on biophysical QC achieved through buffer optimization
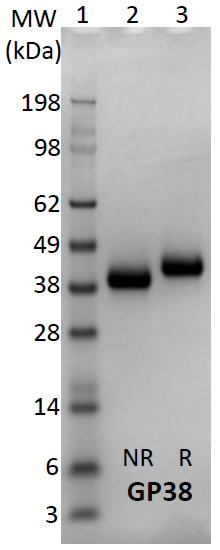
Figure 5. SDS-PAGE gel of 100 % pure GP38 strain A following SEC. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
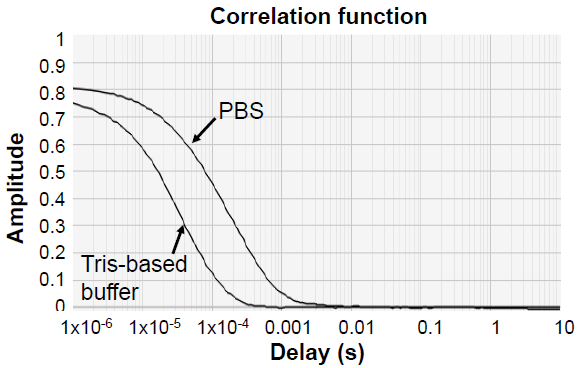
Figure 6. GP38 strain A appeared larger than expected on DLS when in PBS. This resolved to the expected size when buffer-exchanged to a tris-based buffer. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Increased NaCl and imidazole concentrations in affinity buffers prevented non-specific binding of host cell proteins to the column. Tris-based buffer for SEC prevented the reversible buffer-specific oligomerization previously seen in PBS.
Five strains of GP38 were produced to 100 % purity with robust freeze-thaw data
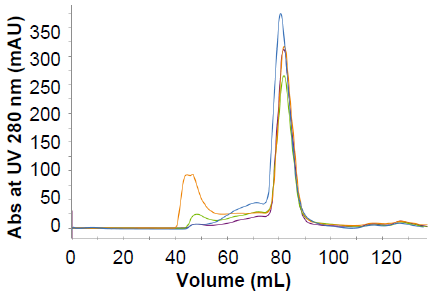
Figure 7. Overlay of preparative SEC chromatogram for strains B-E of GP38. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
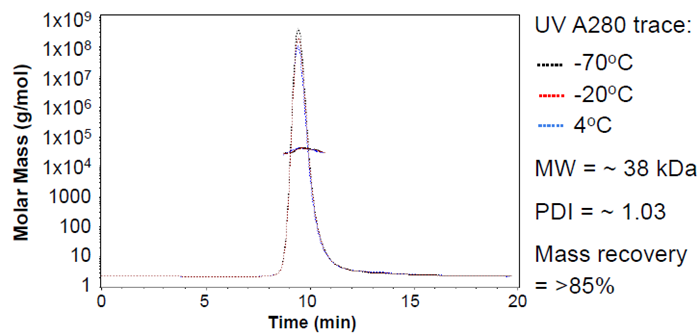
Figure 8. Analysis by SEC-MALS shows GP38 strain B is stable following one cycle of freeze-thaw (either snap-frozen and stored at -70 °C or slow-frozen to -20 °C. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Purified GP38 shows expected binding behavior
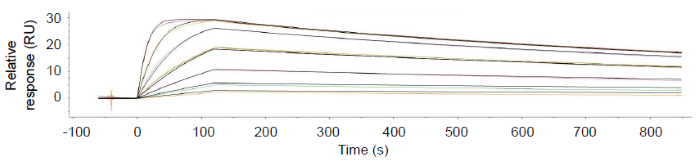
Figure 9. Kinetics profile of chimeric antibody bound to GP38 strain A assessed by surface plasmon resonance fits 1:1 model (KD = 108 pM). This shows that the GP38 is folded in a way that retains good binding to the antibody, and this was also observed for strains B-E. Image Credit: Image courtesy of Amy Harmens et al., in partnership with ELRIG (UK) Ltd.
Discussion
- Production of pure GP38 has enabled antibody humanization to commence.
- Following successful humanization, this antibody could be the first CCHFV treatment to enter clinical trials.
- Biophysical QC results have contributed significantly to our understanding of GP38, particularly regarding the optimal buffers required for the protein’s stability.
References and acknowledgements
- LifeArc. (2025). USAMRIID: A transatlantic collaboration to develop a treatment for Crimean-Congo hemorrhagic fever - LifeArc. (online) Available at: https://www.lifearc.org/project/usamriid-a-transatlantic-collaboration-to-develop-a-treatment-for-cchfv/.
- Hawman, D.W. and Feldmann, H. (2023). Crimean–Congo haemorrhagic fever virus. Nature Reviews Microbiology, (online) 21, pp.1–15. DOI: 10.1038/s41579-023-00871-9. https://www.nature.com/articles/s41579-023-00871-9.
- Mishra, A.K., et al. (2020). Structure and Characterization of Crimean-Congo Hemorrhagic Fever Virus GP38. Journal of Virology, 94(8). DOI: 10.1128/jvi.02005-19. https://journals.asm.org/doi/10.1128/jvi.02005-19.
Thank you to the Protein and Analytical Sciences team led by Beth Wensley, the CCHFV project team led by Rodrigo Abreu, LifeArc’s Global Health Infection team, and USAMRIID collaborators.
About LifeArc
LifeArc® is a medical research charity making life science life-changing, transforming promising life science ideas into medical breakthroughs that change patients’ lives. We are self-funding and specialize in early-stage translation, advancing lab-based scientific discoveries to a point at which they can be developed into the next generation of diagnostics, treatments, and cures. We have been doing this for more than 25 years, and our work has resulted in a diagnostic for antibiotic resistance and four licensed medicines. This includes Keytruda®(cancer), Actemra® (rheumatoid arthritis), Tysabr® (multiple sclerosis), Entyvio® (Crohn’s disease), and a test for antimicrobial resistance.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics, and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programs that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free of charge to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate, and collaborate on an open-access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.Net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Dec 18, 2025