This article is based on a poster originally authored by Rachel Daniels, Yu Chen, Spencer Chiang, Tianfu Zhang and Yuehchun Hsieh.
The in vitro cultivation of immune cells is an important stage in the development of cell and gene treatments. Immune cells, including T, NK, and Macrophages, require unique media to match their diversity and specialized nature.
Each immune cell group has distinct metabolic requirements, activation pathways, and cytokine reliance. Standard, one-size-fits-all media cannot address these features. When studying diverse cell types, specialized media for cell subpopulations are ineffective.
As a result, any adjustments to target immune cell types, such as switching from CAR-T to CAR-NK therapies, typically involve lengthy cell culture optimization processes.
To address these constraints, this study focused on creating a two-part medium: a universal basic medium and custom-formulated supplements tailored and optimized for specific immune cell subpopulations.
This strategy generates an adaptive yet fine-tuned formulation tailored to each cell type's biological and functional needs by carefully adjusting essential media components, including nutrients, cytokines, growth factors, and serum substitutes.
Two supplements, NK cells and yδT cells, were designed to be used with a universal basal medium. The purity of NK cells and yδT cells increased by 1,000 and 17,600 times, respectively, to 97 % and 93 %.
The customizability of each generated cell media allows researchers to readily adopt and execute highly tailored cell media for diverse cell subpopulations, increasing the efficiency of research on different cell types in immune cell therapy studies.
Adaptable cell culture supplement scheme
Image Credit: ACROBiosystems
NK cell media formulation for culturing
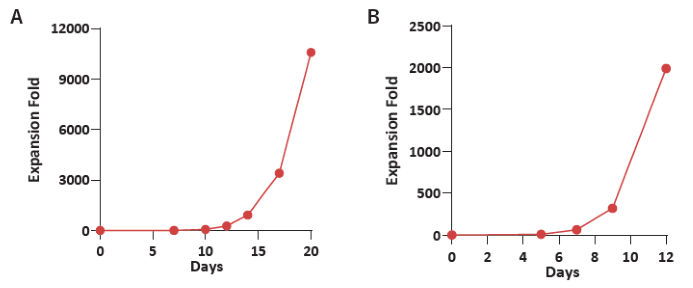
Figure 1. (A) Donor 1 and (B) Donor 2 human CBMCs were cultured using the Immune Cell Basal Medium with CelThera™ Immune Cell Supplement C. The resulting expansion fold exceeds 1,000 on Day 11 and can reach 10,000 on Day 19. Image Credit: ACROBiosystems
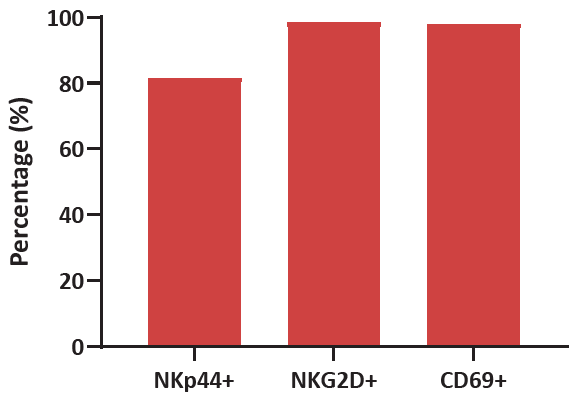
Figure 2. Human CBMCs were cultured for 2 weeks using Immune Cell Basal Medium with CelThera™ Immune Cell Supplement C. Varying ratios of NKp44/NKG2D/CD69, all markers of activation, reveal significant levels of activation after the culturing process. Image Credit: ACROBiosystems
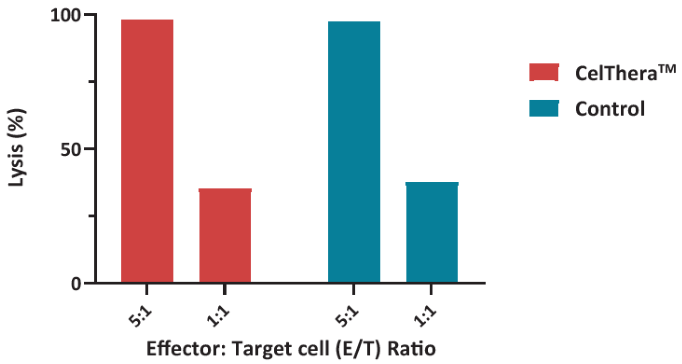
Figure 3. Measurement of remaining target cells was performed after 7-AAD/CFSE staining and analyzed via ELISA. NK cells were used to evaluate cytotoxicity with several E:T ratios, revealing > 30 % of target cells lysed at the lowest ratio, similar to control NK cells. Image Credit: ACROBiosystems
yδT media formulation
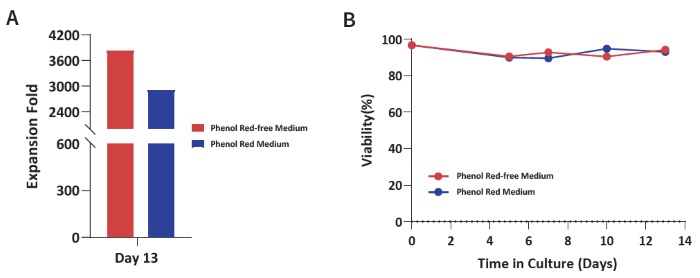
Figure 4. (A) Expansion fold and (B) viability of yδT cells were evaluated from PBMCs cultured with Basal Medium, T cell Expansion Supplement, and GMP Immune Cell Supplement D. Expansion fold exceeds 3,600 with a viability of > 90 % across the culture period. Image Credit: ACROBiosystems
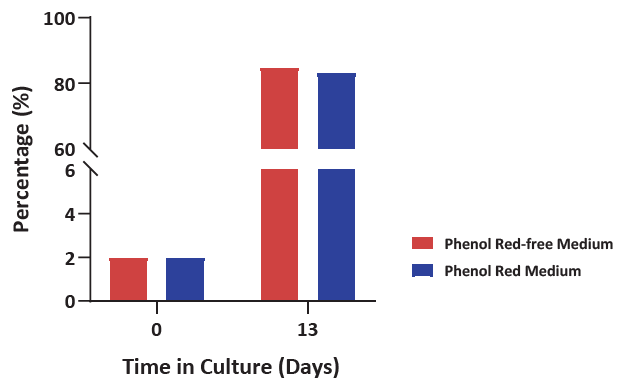
Figure 5. Cell population percentage of yδ2T after culturing using either phenol red or phenol red-free medium resulted in > 80 %. After two weeks, a high percentage of yδ2T was achieved, using the addition of several supplements alongside the traditional basal medium, revealing the capability to tailor media to a specific subpopulation. Image Credit: ACROBiosystems
Conclusion
The creation of a universal basic medium combined with bespoke additives provides a versatile and efficient option for cultivating various immune cell subpopulations.
This technique supports each cell type's particular biological and functional requirements by allowing for exact adjustments of nutrients, cytokines, growth factors, and serum substitutes.
The expansion of NK cells and yδT cells at high purity resulted in 1,000- and 17,600-fold increases, demonstrating their effectiveness. This adaptable platform streamlines the transition between diverse immune cell targets, reducing optimization time and enhancing the scalability of cell and gene therapy studies.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Jan 20, 2026