Introduction
Sources of indoor microplastic exposure
Pathways to the gut
Potential effects on gut health
Evidence from human and animal studies
Reducing indoor microplastic exposure
Conclusions
References
Further reading
Indoor microplastics from textiles, dust, and food can accumulate in the human gut, disrupting microbiota and causing inflammation. Research sindicates thatparticle size, shape, and chemical additives influence risks, but lthe ong-term health effects remain uncertain.
 Image Credit: PixelShot / Shutterstock.com
Image Credit: PixelShot / Shutterstock.com
Introduction
Microplastics (MPs), defined as particles smaller than five millimeters (mm) in diameter, have become global contaminants due to their durability, poor biodegradability, and ability to disperse across air, water, soil, and sediments. MPs accumulate in various ecosystems and have recently been detected in human stool, placenta, and blood.1,5
Outdoor MPs originate from diverse environmental sources; however, indoor concentrations of MPs are often higher and can be shed from textiles, furnishings, and household dust. For example, one study near Seoul reported that indoor MP levels are approximately 1.8 times higher than outdoor levels, at 4.1 and 2.1 particles/m³, respectively.2
Researchers estimate that most people will spend up to 90% of their time indoors. Therefore, inhalation and ingestion through air and dust represent a significant route of chronic MP exposure for humans.2
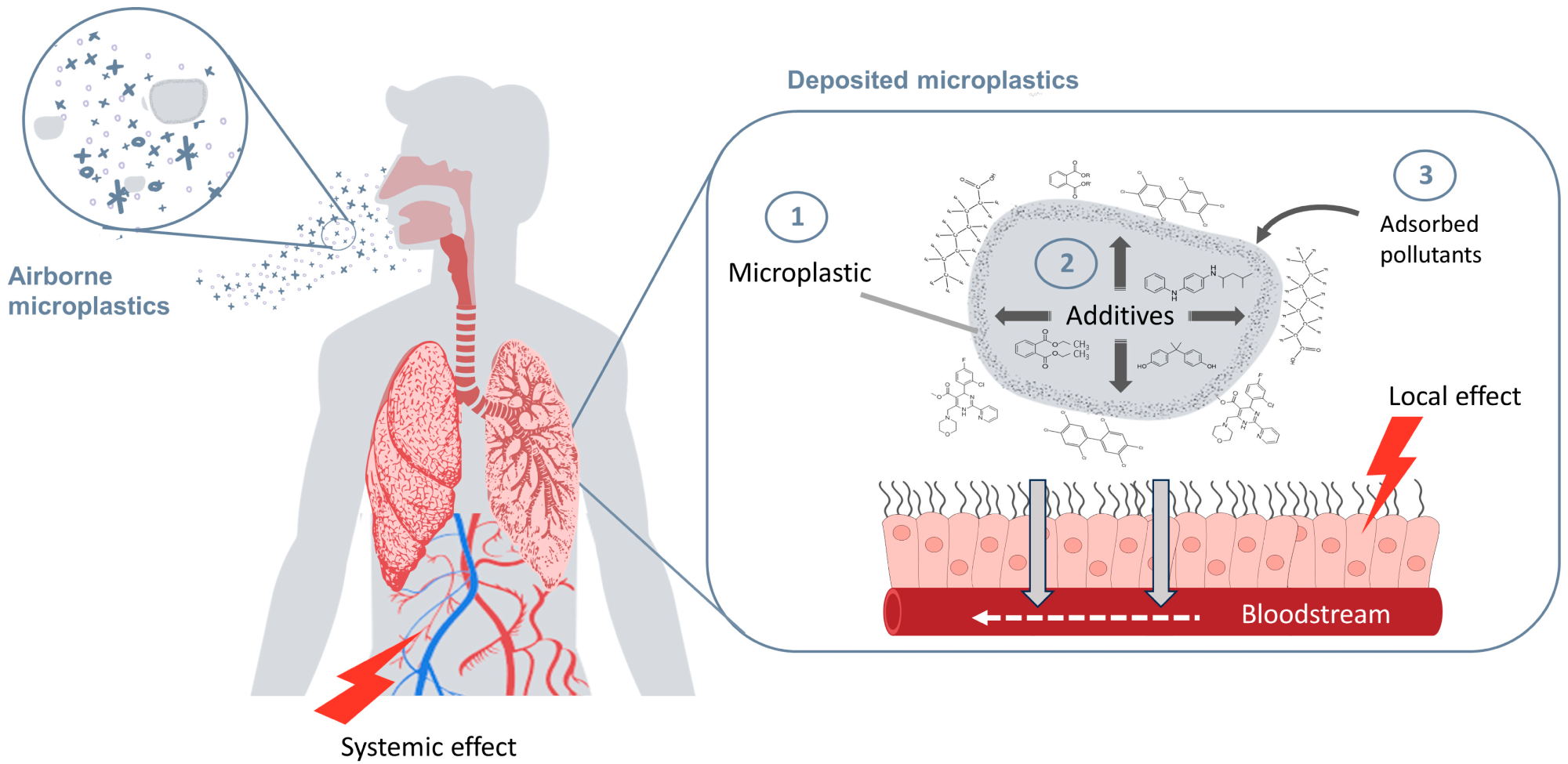
Inhalation exposure to microplastics (MPs) of varying physical and chemical properties. Three situations related to MP deposition in the pulmonary system may harm human health by acting simultaneously or independently. 1. MP particles dare eposited in the lungs. 2. MPs as reservoirs of additives. 3. MPs as carriers of environmental pollutants. This figure was created with https://www.canva.com/.6
Sources of indoor microplastic exposure
Indoor MPs primarily originate from synthetic fibers released from clothing, carpets, upholstery, curtains, and furniture, with carpeting considered a significant source of MPs. Household plastics like flooring, wallpaper, cookware, utensils, and appliance casings also contribute to indoor MP pollution, while fibers and fragments account for nearly 90% of MPs detected indoors.3
Daily activities like vacuuming, walking, and general movement lift settled dust into the air, thereby increasing the risk of exposure by releasing or resuspending particles. Laundering is one of the dprimaryglobal sources of microplastics, with tumble drying also contributing to atherelease. of microplastics into the air indoors Washing outcomes depend strongly on textile composition, yarn structure, and surface treatments in addition to detergent and temperature.4
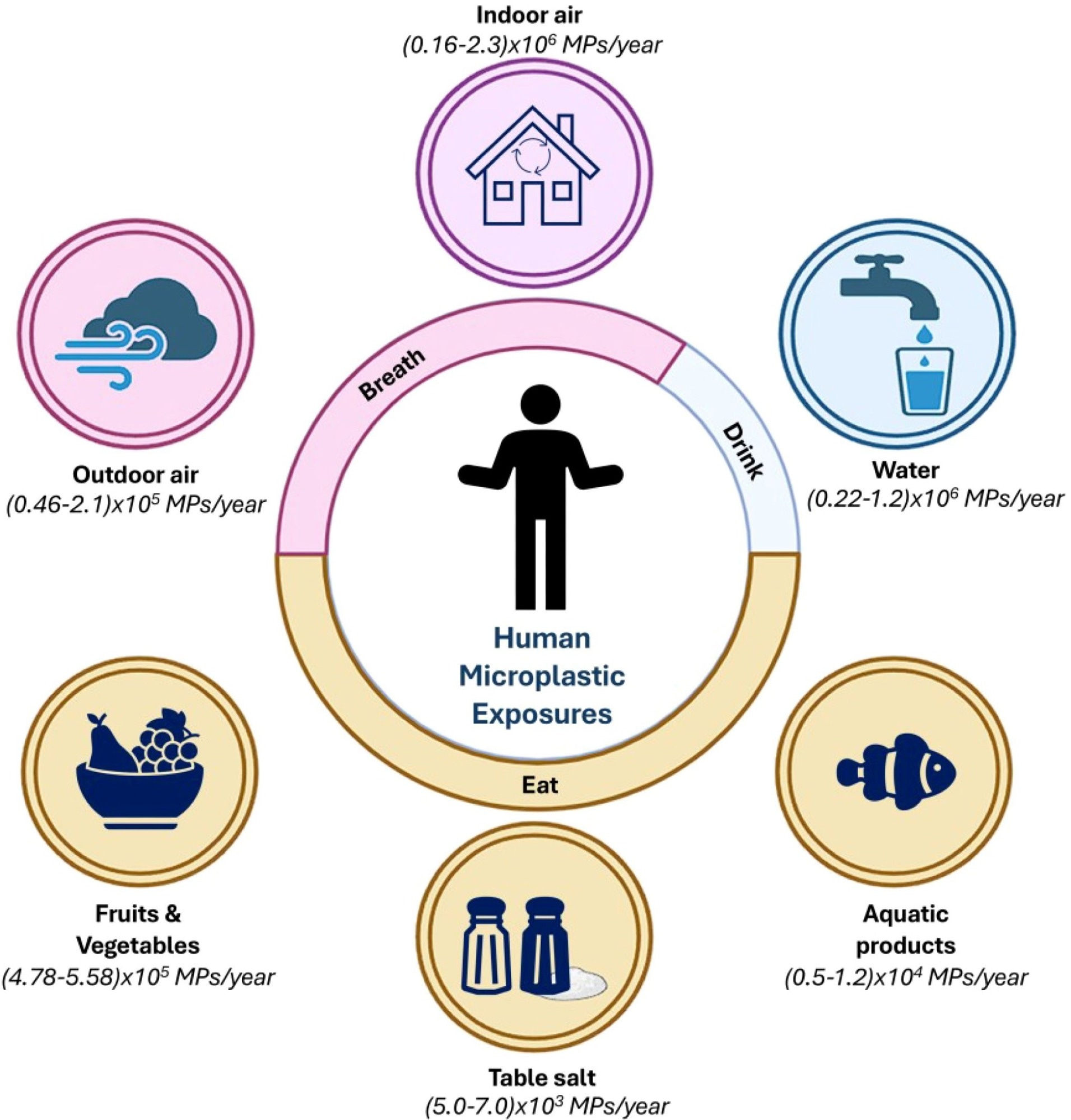
Representative schematic showing the annual intake of microplastics (MPs) by humans through various routes, categorized into breathing (airborne exposure) and eating/drinking (dietary exposure). It gprovidesa comprehensive overview of how dvariousenvironmental sources contribute to the iyearly ngestion or inhalation of MPs yYang et al., 2023). It shows the pervasive presence of MPs in the environment and their inevitable incorporation into human life through daily activities.4
Household dust ingestion is a critical pathway of exposure, particularly for children. Adults may ingest 56 Mmicrograms per ay from dust, whereas pediatric exposure may exceed 100 Mmicrogramsdaily.3
Adjusted for body weight, toddlers are exposed to twelve times greater MP levels than adults at 9.7 and 0.8 MP/kg/day, respectively.3 Frequent hand-to-mouth activity, proximity to floors, and use of plastic bottles or toys further increase the vulnerability of toddlers to MP exposure.1
 Image Credit: SIVStockStudio / Shutterstock.com
Image Credit: SIVStockStudio / Shutterstock.com
Pathways to the gut
Dietary ingestion
Human exposure to MPs occurs primarily through ingestion and inhalation, with some estimates suggesting that humans may ingest between 39,000 and 52,000 MPs annually from diet alone, with higher totals when inhalation is included. Contaminated food, drinking water, and airborne household dust represent the main entry routes.4
MPs have been detected in seafood, salt, honey, and dairy products, reflecting their widespread presence throughout the environment. Both tap and bottled drinking water are also notable MP sources, as wastewater discharge and landfill leachates frequently contaminate urban water systems.4
Airborne exposure
MPs released from synthetic textiles, carpets, and furnishings in households can become airborne and later resettle on surfaces where they are inadvertently ingested during eating or hand-to-mouth contact, especially by children. Occupations involving plastics further increase exposure to airborne MPs.4
Mucociliary clearance and indirect ingestion
The mucociliary escalator, which comprises cilia and mucus that line the airways, transports many of these particles upward to the throat, thereby creating an indirect ingestion route through swallowing. This mechanism, which is otherwise known as mucociliary clearance, represents a substantial but often overlooked pathway by which inhaled MPs ultimately reach the gastrointestinal system.5
Importantly, inhaled MPs and their chemical additives may cross the alveolar-capillary membrane, enter the bloodstream, and accumulate in organs such as the lungs. However, evidence in humans for systemic absorption via inhalation remains limited and debated. The dual role of ingestion and inhalation, followed by mucociliary clearance, exemplifies how MPs can efficiently reach the gut and potentially interact with intestinal tissues and microbiota.6
Potential effects on gut health
MPs can irritate the intestinal lining by causing micro-abrasions that disrupt the tight junctions between epithelial cells, thereby increasing their permeability. Previous studies have reported dose- and size-dependent cytotoxicity, as well as altered mucus production, following MP ingestion, which provides a mechanism for bacterial and antigen translocation to induce both local and systemic immune responses.4
MPs act as vectors of hazardous substances, as they often contain polymer additives such as phthalates, bisphenols, and flame retardants. MPs can also adsorb pollutants, such as heavy metals and persistent organic pollutants (POPs).
For example, beach-deposited MPs can contain up to 2,750 ng/g polychlorinated biphenyl (PCB) and 24,000 ng/g polycyclic aromatic hydrocarbons (PAHs), both of which are chemicals that can leach into the gut, causing oxidative stress, apoptosis, and shifts in gut microbiota.5 MPs may also undergo gastrointestinal biotransformation, form biofilms, and alter key metabolites, such as short-chain fatty acids (SCFAs) and bile acids that are critical for gut homeostasis. Disruption of bile acid metabolism has been linked to microbiota shifts and liver injury in animal models.4
In mice, oral exposure to polyethylene (PE) or polystyrene (PS) MPs causes inflammation, as demonstrated by upregulated toll-like receptor 4 (TLR4), activator protein 1 (AP-1), and interferon regulatory factor 5 (IRF5), as well as reduced levels of regulatory T (Treg) and helper T-cells.7,8 Similar results have been observed in zebrafish and rodent models, with increased reactive oxygen species (ROS)-induced oxidative stress, mitochondrial damage, and histological gut injury observed after MP exposure. Zebrafish studies also reveal metabolic disruptions in lipid and energy pathways.9,10
Invertebrates like mussels also experience impaired antioxidant activity and deoxyribonucleic acid (DNA) damage following MP exposure.11-13 Nanoplastics further suppress immune function, enabling pathogenic bacterial colonization. Increased lipogenic and inflammatory gene expression has also been observed in fish liver, whereas hepatotoxicity, oxidative stress, and fibrosis signals have been reported in human liver organoids and hepatocytes.14 In mice, smaller MPs and nanoplastics penetrate cells and trigger cGAS/STING signaling, leading to liver fibrosis.
A recent systematic review of 28 preclinical studies found consistent evidence of gut dysbiosis. More specifically, MPs increased the levels of Firmicutes, Proteobacteria, and Chlamydia, while decreasing those of Bacteroidetes and γ-Proteobacteria. These changes were accompanied by increased pro-inflammatory cytokines such as IL-1α and TNF-α.15
How microplastics affect your health
Evidence from human and animal studies
Common polymers, including polypropylene (PP), polyethylene terephthalate (PET), PS, and PE, have been identified in human adult stool samples. Higher MP burdens of polycarbonate (PC), polyamide (PA), polyurethane (PU), and PET have been identified in stool samples obtained from children, including meconium from infants.1
A previous study conducted in Xiamen, China, identified PVC, PET, PE, and PA6 in 85.5% of stool samples obtained from preschool children. Notably, children who spent more time eating exhibited higher PVC levels, which is likely due to the propensity of these MPs to precipitate and deposit on food-contact surfaces during prolonged exposure. The same study estimated a median MP intake of 426 µg/kg-bw/day, with a higher risk associated with dairy intake, bottle use, and longer mealtimes.1
Oral exposure to PE or PS MPs in mice leads to dysbiosis, which increases serum D-lactate levels and disrupts the intestinal barrier. In addition to a higher Firmicutes/Bacteroidetes ratio, Actinobacteria species become more abundant following MP exposure. MPs also downregulate genes involved in xenobiotic metabolism and upregulate inflammatory pathways.16,17
Shape and size strongly influence toxicity: fibrous MPs accumulate in the gastrointestinal tract, while fragments cause damage to the jaw and liver, and spherical particles may be expelled more readily. Nanoplastics are particularly potent in reducing organoid viability and altering immune gene expression.
To date, no toxicological thresholds or reference doses have been established between human MP exposure and long-term consequences like carcinogenesis, metabolic disease, or developmental impacts. Therefore, robust longitudinal studies that incorporate real-world exposure levels are necessary to clarify health risks and establish evidence-based safety guidelines. Reviews also highlight possible links to chronic diseases, including cardiometabolic disorders and gut–brain axis effects.5
Reducing indoor microplastic exposure
Ventilation plays a key role in MP exposure, with prolonged or periodic ventilation of 24-48 hours capable of reducing indoor MP concentrations by diluting airborne particles.2 Complementing ventilation, high-efficiency particulate air (HEPA) filters can capture fine particles, including microfibers that are released from carpets, textiles, and household furnishings. Effectiveness depends on filter efficiency, number of units, placement, and airflow conditions.20
Since textiles are primary contributors to indoor MP release, consumers are advised to purchase materials made from natural fibers like cotton, hemp, and linen or tightly woven synthetics. Front-loading machines equipped with filters are ideal, as gentle cycles and cool water have been shown to further reduce fiber shedding.
Broader solutions require regulatory and industrial action. For example, France enacted a requirement that all new washing machines sold from 2025 include microfiber-capturing filters.
Implementing Extended Producer Responsibility (EPR) frameworks can also incentivize manufacturers to design products with reduced shedding and sustainable end-of-life pathways. Mandatory washing machine filters, stricter product labeling, and intentional banning of microplastics in consumer goods represent effective regulatory strategies. Investment in advanced wastewater treatment and material innovation, including biodegradable polymers, can also limit environmental release.4,22
Conclusions
The ingestion of MPs through household dust, contaminated food, and inhaled particles cleared through mucociliary pathways may contribute to gastrointestinal disruption. Experimental and epidemiological studies demonstrate that MPs can induce inflammation, alter the gut microbiome, and act as carriers for harmful chemicals, all of which increase the risk of adverse, long-term health effects.
To clarify the health significance, there remains an urgent need for well-designed and region-specific studies that accurately quantify indoor exposure, assess bioaccumulation, and evaluate causal links to gastrointestinal outcomes. Targeted strategies to mitigate exposure and protect public health.
References
- Ke D et al. (2023). Occurrence of microplastics and disturbance of gut microbiota: a pilot study of preschool children in Xiamen, China. EBioMedicine.;97:104828. DOI:10.1016/j.ebiom.2023.104828, https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(23)00394-8/fulltext
- Jahanzaib, M., Sharma, S., & Park, D. (2025). Microplastics comparison of indoor and outdoor air and ventilation rate effect in outskirts of the Seoul metropolitan city. Emerging Contaminants, 11(1), 100408. DOI:10.1016/j.emcon.2024.100408, https://www.sciencedirect.com/science/article/pii/S2405665024001094.
- Ageel, H. K., Harrad, S., & Abdallah, M. A. E. (2025). Microplastics in settled indoor dust: Implications for human exposure. Emerging Contaminants, 11(3), 100506. DOI:10.1016/j.emcon.2025.100506, https://www.sciencedirect.com/science/article/pii/S240566502500040X.
- Bora, S. S., et al. (2024). Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Frontiers in Cellular and Infection Microbiology, 14, 1492759. DOI:10.3389/fcimb.2024.1492759, https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2024.1492759/full.
- Hirt, N., Body-Malapel, M. (2020). Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol, 17; 57. DOI:10.1186/s12989-020-00387-7, https://particleandfibretoxicology.biomedcentral.com/articles/10.1186/s12989-020-00387-7.
- Borgatta, M., & Breider, F. (2024). Inhalation of Microplastics—A Toxicological Complexity. Toxics 12(5), 358. DOI:10.3390/toxics12050358, https://www.mdpi.com/2305-6304/12/5/358
- Li, B. et al. (2020). Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere, 244:125492, DOI:10.1016/j.chemosphere.2019.125492, https://www.sciencedirect.com/science/article/abs/pii/S0045653519327328.
- Park E-J et al. (2020). Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicology Letters 324:75-85. DOI:10.1016/j.toxlet.2020.01.008, https://www.sciencedirect.com/science/article/abs/pii/S0378427420300163.
- Shen, R., et al. (2022). Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environmental Pollution 300; 118986. DOI:10.1016/j.envpol.2022.118986, https://www.sciencedirect.com/science/article/abs/pii/S0269749122002007.
- Qiao, R., Sheng, C., Lu, Y., et al. (2019). Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Science of the Total Environment 662;246-253. DOI:10.1016/j.scitotenv.2019.01.245, https://www.sciencedirect.com/science/article/abs/pii/S0048969719303006.
- Brandts, I., et al. (2018). Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci Total Environ., 643:775-784, DOI:10.1016/j.scitotenv.2018.06.257, https://www.sciencedirect.com/science/article/abs/pii/S0048969718323337.
- Paul-Pont, I., et al. (2016). Exposure of marine mussels Mytilus spp. to polystyrene microplastics: toxicity and influence on fluoranthene bioaccumulation. Environmental Pollution 216:724-737. DOI:10.1016/j.envpol.2016.06.039, https://www.sciencedirect.com/science/article/abs/pii/S0269749116305279.
- Auguste, M., Lasa, A., Balbi, T., et al. (2020). Impact of nanoplastics on hemolymph immune parameters and microbiota composition in Mytilus galloprovincialis. Mar Environ Res., 159:105017, DOI:10.1016/j.marenvres.2020.105017, https://www.sciencedirect.com/science/article/abs/pii/S0141113620301483.
- Li, Y., Chen, L., Zhou, N., et al. (2024). Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity. Sci. Total Environ. 946, 174215. DOI:10.1016/j.scitotenv.2024.174215, https://www.sciencedirect.com/science/article/abs/pii/S0048969724043638.
- Souza-Silva, T. G. D. et al. (2022). Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence. Life Sciences 294, 120366, DOI:10.1016/j.lfs.2022.120366, https://www.sciencedirect.com/science/article/pii/S0024320522000662.
- Deng, Y., et al. (2020). Microplastics Release Phthalate Esters and Cause Aggravated Adverse Effects in the Mouse Gut. Environ. Int., 143, 105916, DOI:10.1016/j.envint.2020.105916, https://www.sciencedirect.com/science/article/pii/S0160412020318717.
- Jiang, P., et al. (2021). Effects of Microplastics (MPs) and Tributyltin (TBT) Alone and in Combination on Bile Acids and Gut Microbiota Crosstalk in Mice. Ecotoxicol. Environ. Saf., 220, 112345, DOI:10.1016/j.ecoenv.2021.112345, https://www.sciencedirect.com/science/article/pii/S0147651321004565.
- Park, S. B., Jung, W. H., Choi, K. J., et al. (2023). A Comparative Systematic Analysis of The Influence of Microplastics on Colon Cells, Mouse, and Colon Organoids. Tissue Eng. Regen. Med., 20; 49-58, DOI:10.1007/s13770-022-00496-8, https://link.springer.com/article/10.1007/s13770-022-00496-8
- Jabeen, K. et al. (2018). Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere, 213, 323-332. DOI:10.1016/j.chemosphere.2018.09.031, https://www.sciencedirect.com/science/article/abs/pii/S0045653518316849.
- Chen, C., Hsu, C., Chang, Y., et al. (2021). Efficacy of HEPA Air Cleaner on Improving Indoor Particulate Matter 2.5 Concentration. International Journal of Environmental Research and Public Health, 19(18), 11517. DOI:10.3390/ijerph191811517, https://www.mdpi.com/1660-4601/19/18/11517.
- Gliaudelytė, U., Persson, M., & Daukantienė, V. (2024). Impact of textile composition, structure, and treatment on microplastic release during washing: A review. Textile Research Journal. DOI:10.1177/00405175241260066, https://journals.sagepub.com/doi/10.1177/00405175241260066
- Gaylarde, C., Baptista-Neto, J. A., & Da Fonseca, E. M. (2021). Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 7(5), e07105. DOI:10.1016/j.heliyon.2021.e07105, https://www.sciencedirect.com/science/article/pii/S2405844021012081.
Further Reading
Last Updated: Aug 31, 2025