Introduction
What is protein restriction (PR)?
Evidence from animal studies
Modern PR diets
Practical patterns
Potential risks
Summary
References
Further reading
Protein restriction may influence longevity by modulating nutrient-sensing pathways and improving metabolic health, especially when applied intermittently. Age, health status, and protein quality remain crucial factors in safely implementing low-protein dietary strategies.
 Image Credit: margouillat photo / Shutterstock.com
Image Credit: margouillat photo / Shutterstock.com
Introduction
This article discusses the different mechanisms through which the protein restriction (PR) diet promotes longevity while navigating its age-dependent risks and benefits.
What is protein restriction (PR)?
Calorie restriction (CR), which involves a 10-30% reduction in caloric intake without resulting in malnutrition, has been widely used to delay aging and increase longevity.1 Although CR is associated with powerful protective effects against a wide range of age-related diseases, its requirement for chronic, significant reductions in energy intake remains a challenge for human adherence.2
The quantity, source, and composition of dietary protein may be more strongly associated with healthspan and longevity than calorie intake alone.2 Consequently, PR, a comparatively novel principle, has emerged as a more targeted and potentially translatable strategy.1,2
Numerous studies demonstrate that lower protein intake during midlife can activate molecular pathways linked to longevity, while clinical guidelines for older adults emphasize the critical need for higher protein intake to prevent sarcopenia.3 The effects of dietary protein on aging are mediated by a highly conserved network of nutrient-sensing molecular pathways.
At the center of this network is the mechanistic target of rapamycin (mTOR), a protein kinase that acts as a master regulator of cell growth and metabolism.4 The mTOR pathway, particularly the mTORC1 complex, is potently activated by amino acids, insulin, and growth factors like insulin-like growth factor 1 (IGF-1).5 When activated, mTORC1 promotes anabolic processes like protein synthesis while simultaneously inhibiting catabolic processes, particularly autophagy.4
While essential for growth, chronic mTORC1 activation is a key driver of aging. Consequently, its inhibition, either genetically or with drugs like rapamycin, has consistently extended lifespan in mouse models, although evidence for lifespan extension in non-human primates remains limited and is better characterized as improvements in healthspan rather than confirmed survival benefits.4
Evidence from animal studies
In rodent models, reducing dietary protein from typical levels of about 20% of total caloric intake to 5–10% increases maximum lifespan by over 20%.5 More targeted interventions have revealed similarly potent effects, as demonstrated by the restriction of a single essential amino acid, methionine, which extended the maximum lifespan of rodents by substantial amounts, though precise upper estimates vary across studies and model systems.6
Methionine-based interventions have been shown to improve metabolic health markers, including lower serum IGF-1, insulin, and glucose levels. These effects were also accompanied by improvements in oxidative-stress resistance and slower age-related immune and lens changes.6 The profound effect of methionine restriction suggests that specific amino acids may act as potent signaling molecules and that the benefits of general PR may be due to the reduction of these key components.5,6
Methionine Restriction as a Life Extension Strategy
Modern PR diets
The traditional diet of the Okinawan people, a Japanese population widely recognized for its longevity, provides a compelling real-world example of how integrating epidemiological observations with clinical trial data can translate findings from animal models into humans.5
Traditional Okinawan diets were historically very low in protein and high in carbohydrates, with 9% and 85% of total caloric intake, respectively. Similarly, the traditional Mediterranean diet, which is also associated with lower mortality and healthy aging, features a low protein-to-carbohydrate ratio.7
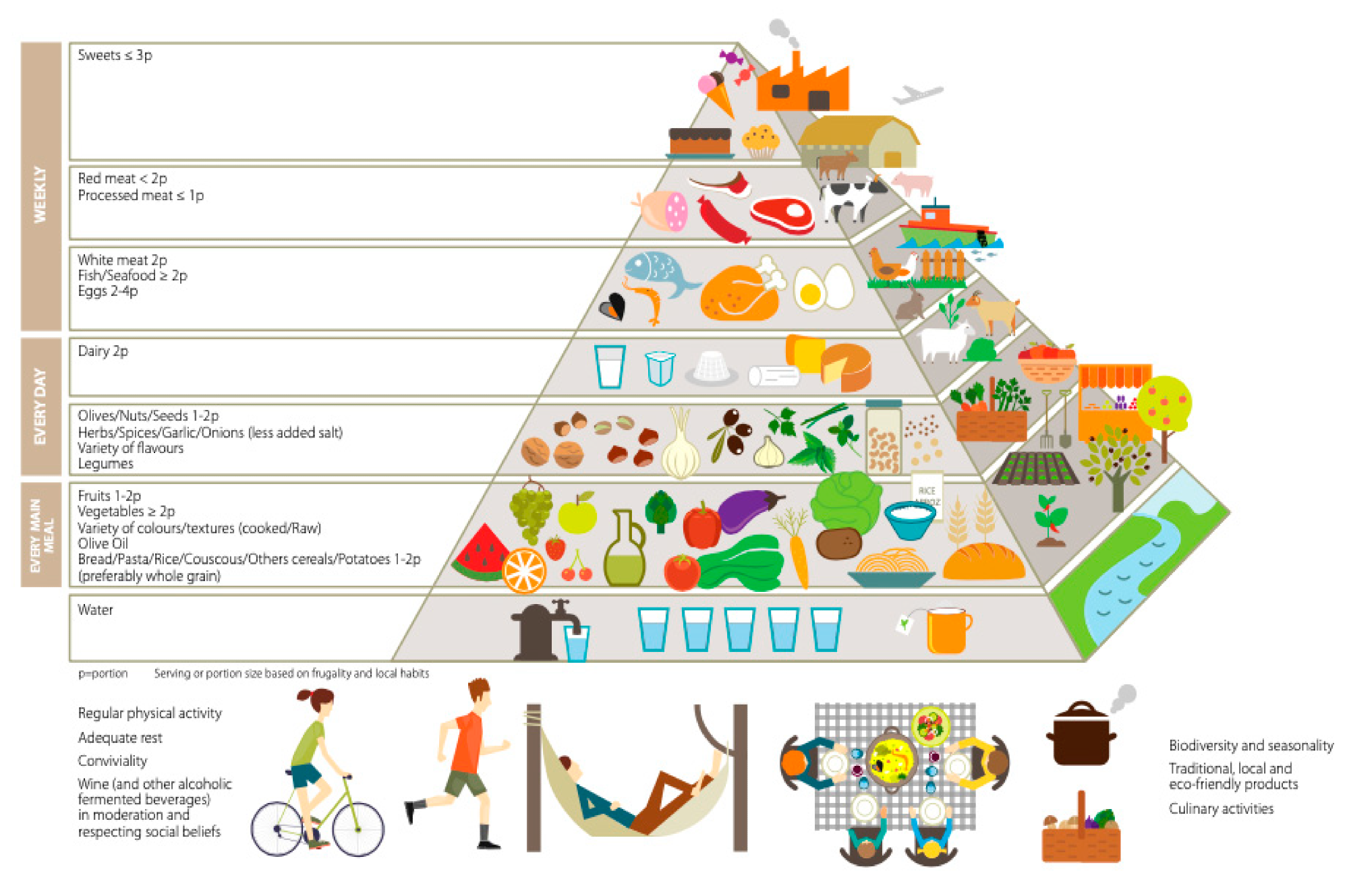
Pyramid for a Sustainable Mediterranean Diet.
The five-day Fasting-Mimicking Diet (FMD), which is also low in calories and protein, supports weight loss, healthy blood pressure, and reduced circulating IGF-1 levels in healthy adults. These changes reflect short-term improvements in metabolic risk factors rather than direct evidence of lifespan extension in humans.8
A large 2020 meta-analysis of prospective cohort studies suggests that higher intake of plant protein is associated with an 8% reduced risk of all-cause mortality and a 12% reduced risk of cardiovascular mortality. This association was dose-responsive, with each 3% increase in energy from plant protein linked to a 5% lower risk of all-cause mortality. The same analysis also found that higher total protein intake was modestly associated with lower all-cause mortality, while animal protein intake showed no clear association, highlighting the importance of protein source rather than absolute protein quantity.9
Practical patterns
The risks associated with chronic protein deficiency, particularly for older adults, may improve the safety profile and feasibility of intermittent or cyclical restriction rather than continuous PR. The success of the FMD protocol suggests that brief, periodic cycles of restriction can lead to beneficial metabolic reprogramming without compromising long-term nutritional status.8
A practical application could involve implementing one or two ‘low-protein days’ every week, during which protein intake is consciously reduced to levels below 0.8 g/kg body weight. Emphasizing plant-based sources of protein like legumes, tofu, quinoa, and nuts over animal-derived proteins, particularly red and processed meats, supports longevity.9 This approach may confer a dual benefit, as plant proteins are generally lower in methionine, partially mimicking the effects of targeted methionine restriction observed in animal models.2
Potential risks
The primary risk of inappropriate PR is sarcopenia, an age-related loss of muscle mass and strength. Older adults are at a greater risk of experiencing anabolic resistance, an impaired muscle-building response to protein intake, which necessitates higher dietary protein intake to maintain muscle.10
Public health agencies recommend that healthy older adults consume at least 1.0–1.2 g/kg/day, with higher amounts (1.2–1.5 g/kg/day) advised for those with chronic disease. These recommendations are supported by both European ESPEN guidelines and Korean geriatric consensus statements. Rather than directly opposing all forms of PR, these guidelines indicate that chronic or severe PR is inappropriate for older adults, while carefully structured intermittent approaches may still be feasible if adequate protein intake is maintained during non-restriction days.3,10
Chronic, severe PR can also lead to fatigue, impaired immune function, and micronutrient deficiencies. As a result, PR is contraindicated for high-risk populations, including the frail elderly, pregnant or lactating women, children, and individuals recovering from illness or surgery. Additionally, individuals with existing kidney disease or at high risk for chronic kidney disease should avoid high-protein diets, as excessive protein intake may contribute to kidney stress; however, PR must still be balanced to prevent malnutrition.3
Summary
Modulating protein intake rather than just calories is a powerful way to positively impact the molecular pathways implicated in aging. Chronic PR is neither practical nor safe for most people; however, a nuanced approach that considers age, kidney health, and metabolic status has the potential to confer significant health benefits.
For healthy middle-aged adults, periodic cycles of low protein intake, combined with a lifelong dietary pattern that emphasizes plant-based protein sources, may safely confer many of the metabolic benefits observed in longevity research across human and non-human models. This strategy could activate key cellular maintenance programs, such as autophagy, and improve metabolic health by reducing IGF-1 and mTOR signaling, while recognizing that definitive evidence of increased human lifespan is not yet available.
References
- Ferraz-Bannitz, R., Beraldo, R. A., Peluso, A. A., et al. (2022). Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients 14(13); 2670. DOI:10.3390/nu14132670, https://www.mdpi.com/2072-6643/14/13/2670.
- Ko, G., Rhee, C. M., Kalantar-Zadeh, K., & Joshi, S. (2020). The Effects of High-Protein Diets on Kidney Health and Longevity. Journal of the American Society of Nephrology 31(8); 1667-1679. DOI:10.1681/ASN.2020010028, https://journals.lww.com/jasn/abstract/2020/08000/the_effects_of_high_protein_diets_on_kidney_health.7.aspx.
- Deutz, N. E. P., Bauer, J. M., Barazzoni, R., et al. (2014). Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clinical Nutrition 33(6); 929-936. DOI:10.1016/j.clnu.2014.04.007, https://www.clinicalnutritionjournal.com/article/S0261-5614(14)00111-3/fulltext.
- Madeo, F., Zimmermann, A., Maiuri, M. C., & Kroemer, G. (2015). Essential role for autophagy in life span extension. Journal of Clinical Investigation 125(1); 85-93. DOI:10.1172/JCI73946, https://www.jci.org/articles/view/73946.
- Willcox, D. C., Scapagnini, G., & Willcox, B. J. (2014). Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mechanisms of Ageing and Development. DOI:10.1016/j.mad.2014.01.002, https://www.sciencedirect.com/science/article/abs/pii/S0047637414000037.
- Miller, R. A., Buehner, G., Chang, Y., Harper, J. M., Sigler, R., & Smith‐Wheelock, M. (2005). Methionine‐deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF‐I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4(3); 119–125. DOI:10.1111/j.1474-9726.2005.00165.x, https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1474-9726.2005.00152.x.
- Serra-Majem, L., Tomaino, L., Dernini, S., et al. (2020). Updating the Mediterranean Diet Pyramid towards Sustainability: Focus on Environmental Concerns. International Journal of Environmental Research and Public Health 17(23); 8758. DOI:10.3390/ijerph17238758, https://www.mdpi.com/1660-4601/17/23/8758.
- Wei, M., Brandhorst, S., Shelehchi, M., et al. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Science Translational Medicine 9(377). DOI:10.1126/scitranslmed.aai8700, https://www.science.org/doi/10.1126/scitranslmed.aai8700.
- Naghshi, S., Sadeghi, O., Willett, W. C., & Esmaillzadeh, A. (2020). Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. DOI:10.1136/bmj.m2412, https://www.bmj.com/content/370/bmj.m2412.
- Jung, H., Kim, S., Kim, I., et al. (2018). Protein Intake Recommendation for Korean Older Adults to Prevent Sarcopenia: Expert Consensus by the Korean Geriatric Society and the Korean Nutrition Society. Annals of Geriatric Medicine and Research 22(4); 167-175. DOI:10.4235/agmr.18.0046, https://www.e-agmr.org/journal/view.php?doi=10.4235/agmr.18.0046.
Further Reading
Last Updated: Nov 20, 2025