Introduction
The male reproductive microbiome
Mechanisms linking microbiome and fertility
Evidence from research studies
Clinical implications
Challenges and knowledge gaps
Conclusions
References
Further reading
This article explores how the male reproductive microbiome influences fertility through mechanisms such as inflammation, oxidative stress, and hormonal disruption. It also highlights clinical implications, including diagnostic profiling and probiotic therapies that may improve semen quality and ART outcomes.
 Image Credit: Anusorn Nakdee / Shutterstock.com
Image Credit: Anusorn Nakdee / Shutterstock.com
Introduction
Over the past 30 years, male infertility rates have increased by nearly 75%, with current estimates suggesting that this condition affects over 56 million men throughout the world.1,2 Despite decades of research, 30-70% of male infertility cases are classified as idiopathic.1 Male factor infertility contributes to approximately half of all infertility cases worldwide, and oxidative stress, infections, and microbial dysbiosis are increasingly recognized as contributors alongside genetic and endocrine causes.3,6,11
The male reproductive microbiome
The male reproductive tract is a specialized environment that is inhabited by a diverse microbiota. Semen, which contains secretions from multiple glands and the testes, serves as a convergence point for region-specific bacterial communities and, as a result, is considered representative of the entire male reproductive microbiome.3,4
Next-generation sequencing (NGS) technologies, such as genome sequencing and high-throughput proteomics, have enabled the identification of a diverse and complex microbial community within the semen, testes, and urogenital tract. These technological advances, combined with the widespread availability of patient datasets, demonstrate that the seminal microbiome of healthy men is typically dominated by Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes.4
Eubiosis, which is characterized by the predominance of beneficial bacteria like Lactobacillus, is increasingly associated with higher sperm quality and a reduced risk of prostatitis.4 Comparatively, dysbiosis is more frequently observed in infertile men and is accompanied by poor semen parameters. Distinct semen microbiome clusters dominated by Lactobacillus, Prevotella, or Streptococcus have been identified, with the Lactobacillus-dominant cluster associated with the best semen quality and fertility outcomes.5
The overgrowth of Prevotella, Ureaplasma, and Mycoplasma is also a common feature of dysbiosis, all of which are pathogens that have been implicated in adverse fertility outcomes.3 For example, Ureaplasma parvum has been shown to impair sperm motility and morphology, though targeted antibiotic treatment can reverse these effects. Increased inflammation and oxidative stress, both of which are known risk factors in male infertility, often occur in the presence of these microorganisms, which can further impact fertility outcomes.8
Mechanisms linking microbiome and fertility
An imbalance in the seminal microbiota can directly impair sperm quality, as observed in animal and preclinical models. Pathogenic or opportunistic bacteria subsequently adhere to spermatozoa, which leads to agglutination and reduced motility.1,5 These microorganisms can also induce an inflammatory response characterized by leukocyte infiltration into the semen, which releases high levels of reactive oxygen species (ROS).
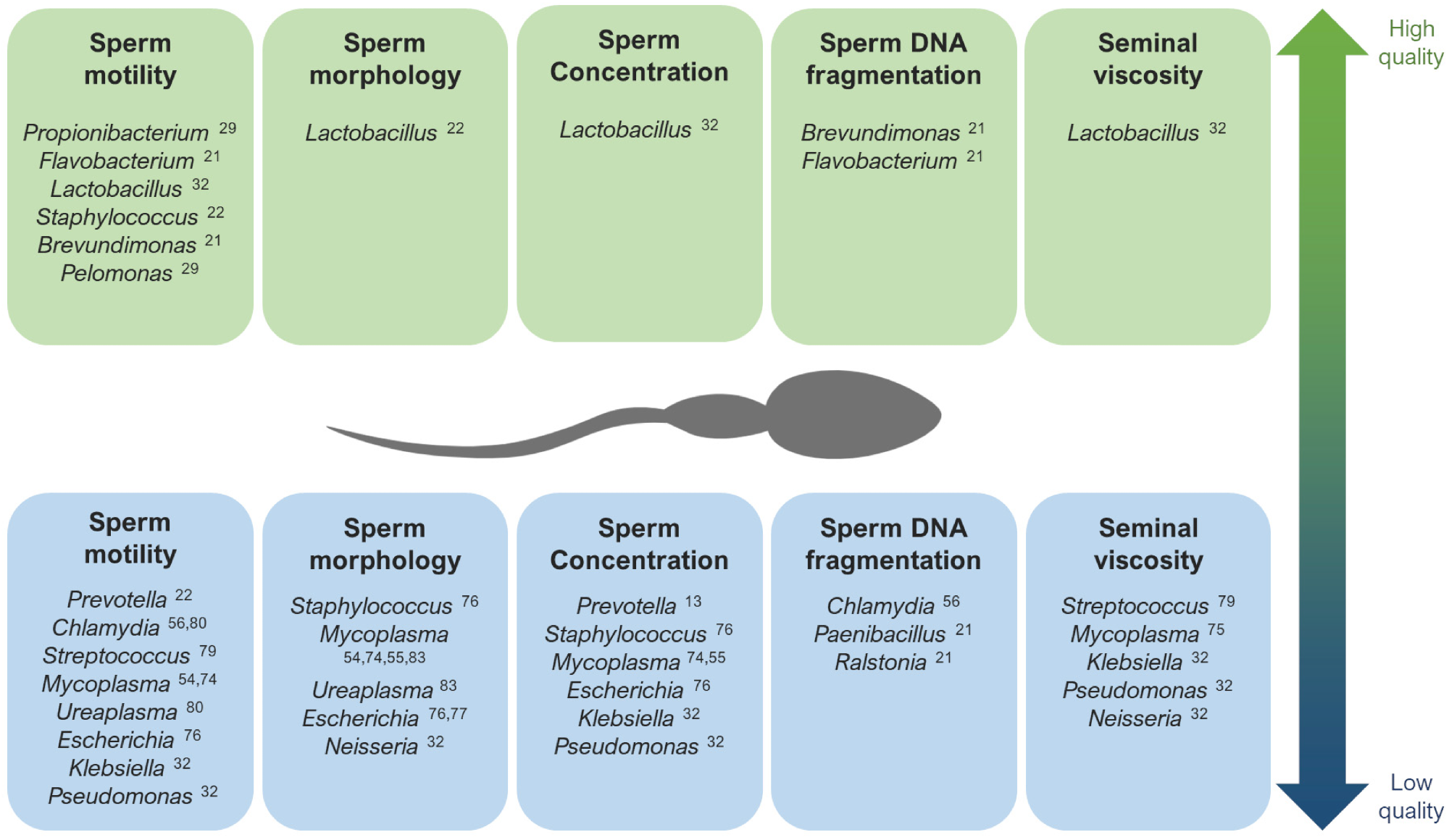
The main genera that have been described as potentially associated with high quality (up) and low quality (down) seminal parameters. 1
Unlike most somatic cells, sperm have minimal antioxidant defenses. As a result, ROS infiltration can lead to lipid peroxidation of sperm membrane and DNA fragmentation, both of which are strongly associated with reduced fertility and poor embryo development.1,5 ROS-driven DNA fragmentation is a consistent finding in infertile men and is reduced after interventions such as probiotics that boost antioxidant defenses.11
The gut-testis axis is a bidirectional communication pathway that connects the intestinal microbiome to testicular function.6 Dysbiosis has been observed to disrupt this axis by compromising the integrity of the intestinal barrier, which allows bacterial endotoxins like lipopolysaccharide (LPS) to enter the bloodstream.7
Systemic endotoxemia leads to a chronic, low-grade inflammatory state, which causes testicular inflammation and impairs spermatogenesis. This inflammation can also suppress the hypothalamic-pituitary-gonadal (HPG) axis, negatively impacting testosterone production.1,6,7 Mouse studies confirm that the absence of gut microbiota reduces testicular size, alters epididymal immune signaling, and transmits reproductive dysfunction to offspring, providing causal evidence for the gut–testis axis.9
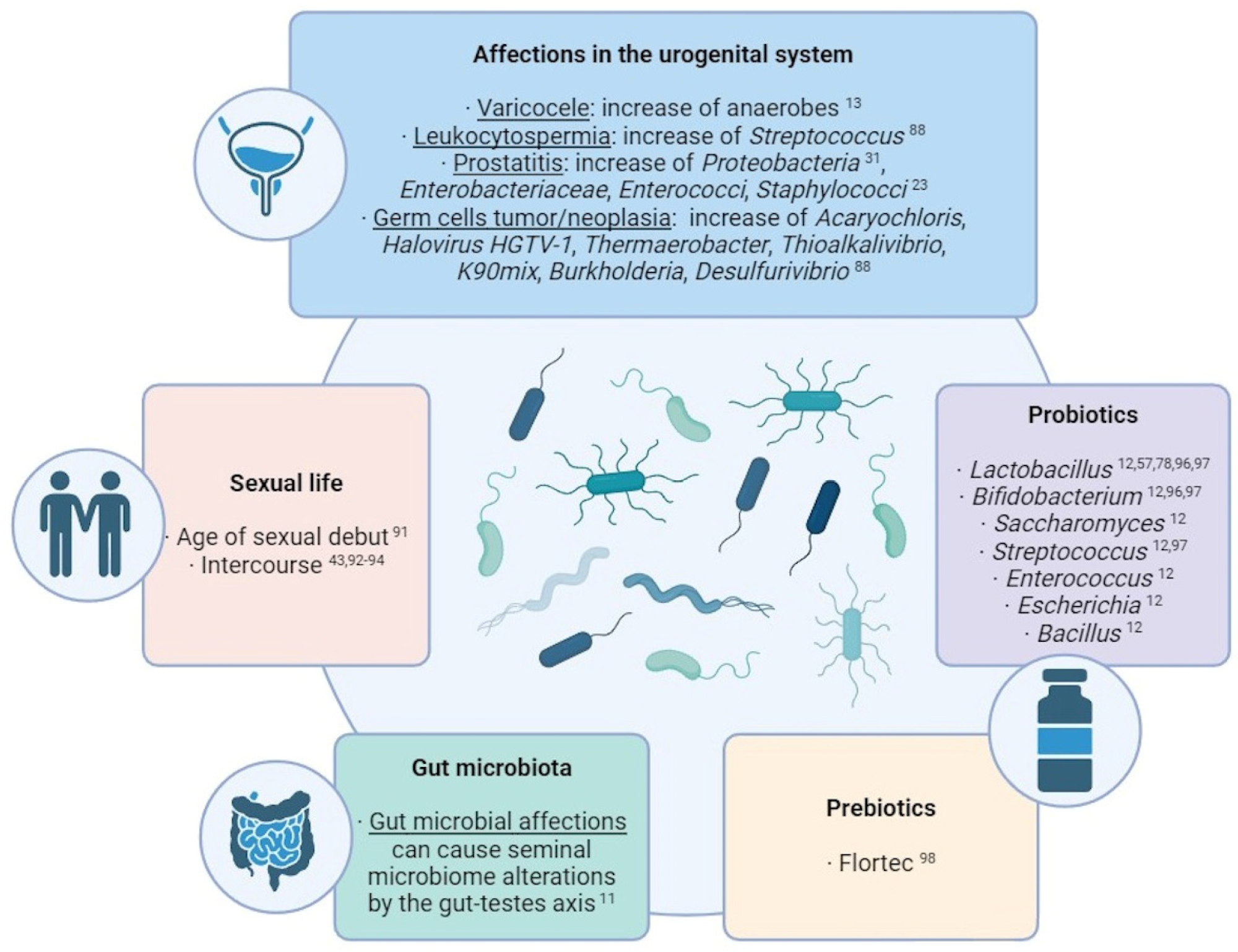
Summary of the main external factors with an influence over the seminal microbiome composition.1
Evidence from research studies
Research has consistently reported an association between the overgrowth of certain bacteria and poor semen parameters. For example, the presence of Ureaplasma in semen contributes to epigenetic changes in sperm and reduced motility, potentially through the production of cytotoxic ammonia and induction of oxidative stress.3,4,8
To date, most human studies have been correlational, whereas preclinical research provides more definitive evidence for a causal relationship between gut dysbiosis and male infertility.9 In germ-free mouse models, mice lacking a gut microbiome exhibited decreased testicular weight and signs of epididymitis-like inflammation as compared to wildtype mice, thereby providing causal evidence that the presence of a healthy microbiome is essential for normal male reproductive tract development and function.8,9
Large-scale metagenomic studies have further shown that semen microbiome composition can distinguish between fertile and infertile men, suggesting potential diagnostic utility.5
Table 1. Microbial Associations with Male Fertility
| Microbe |
Association |
Effect on Fertility |
Reference |
| Lactobacillus |
Positive |
Improved semen quality, higher IVF success |
3,4,5,10 |
| Faecalibacterium |
Positive |
Enriched in successful IVF samples |
10 |
| Prevotella |
Negative |
Poor semen parameters, failed ART cycles |
3,5,10 |
| Anaerococcus |
Negative |
Associated with reduced semen quality |
4 |
| Ureaplasma parvum |
Negative |
Impaired motility & morphology; reversible with antibiotics |
8 |
| Mycoplasma |
Negative |
Linked to inflammation and oxidative stress |
3 |
Table 2. Effects of Probiotic Supplementation on Male Fertility
| Study |
Probiotic Strains |
Main Outcomes |
Reference |
| Valcarce et al. (2017) |
L. rhamnosus, B. longum |
↑ Motility, ↓ DNA fragmentation |
12 |
| Maretti & Cavallini (2017) |
L. paracasei + prebiotics |
↑ Volume, motility, morphology, hormones |
13 |
| Helli et al. (2022) |
Multi-strain (7 bacteria) |
↑ Count, motility, antioxidant capacity; ↓ CRP, TNF-α |
14 |
| Abbasi et al. (2021) |
Multi-strain + prebiotics |
↑ Concentration, motility, morphology; ↓ lipid peroxidation |
15 |
Clinical implications
Researchers are currently investigating the diagnostic potential of microbiome profiling for semen through 16S ribosomal ribonucleic acid (rRNA) sequencing to identify potentially dysbiotic microbial signatures correlated with idiopathic male infertility.10
In fact, the composition of the seminal microbiome has already been used to predict outcomes in assisted reproductive technologies (ART). In one study on in vitro fertilization (IVF), semen samples from successful cycles were significantly enriched with Lactobacillus jensenii and Faecalibacterium, whereas samples from failed cycles had a higher abundance of Prevotella and Bacteroides.10
A systematic review of randomized clinical trials (RCTs) has also reported that oral probiotic supplementation, typically with Lactobacillus and Bifidobacterium strains, significantly improves sperm concentration, motility, and morphology.11
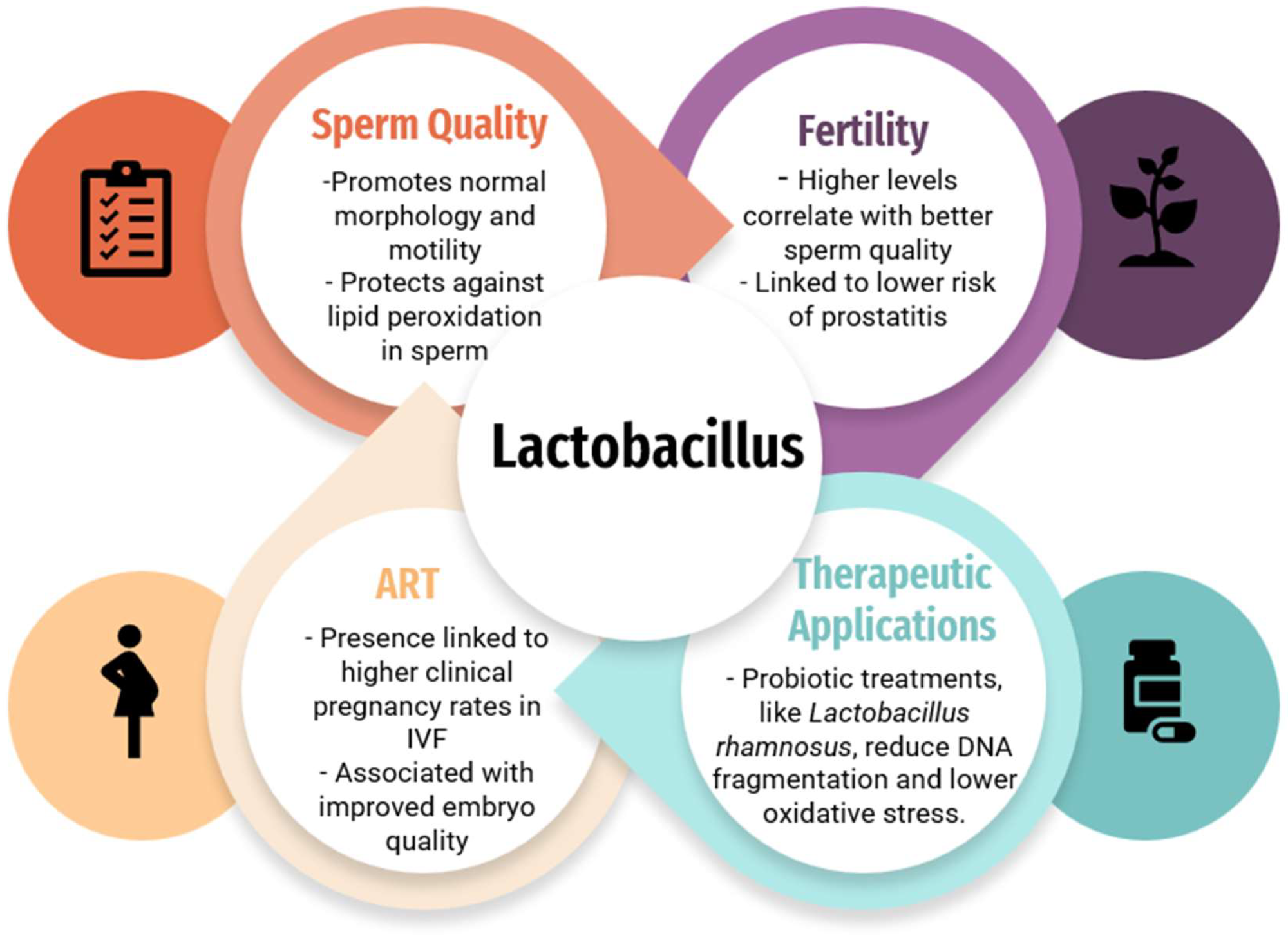
The impact of the Lactobacillus species on fertility, sperm quality, and ART outcomes, and therapeutic applications of the Lactobacillus species.3
Probiotic use was also associated with reduced sperm DNA fragmentation, improved total antioxidant capacity, and decreased inflammatory markers such as CRP and TNF-α, supporting their potential as adjunct therapies for idiopathic infertility.12–15
Challenges and knowledge gaps
The primary challenge encountered in male reproductive microbiome research is that most human studies are cross-sectional and have established correlation, rather than causation. The lack of standardized protocols for sample collection, DNA extraction, sequencing, and data analysis further limits the generalizability of any clinical observations.3,4
Sample contamination and inadequate patient sample sizes also remain a challenge, particularly due to the low microbial biomass in reproductive samples.1 Another limitation is variability across geographic regions and sequencing methods, which complicates comparisons between studies and hinders meta-analysis.3,5
Fertility crisis: Is modern life making men infertile? - BBC REEL
Conclusions
The male reproductive microbiome has emerged as a crucial and previously overlooked factor in male reproductive health. Although additional human trials must be conducted before causation can be established, inflammation, oxidative stress, and endocrine modulation are known mechanisms that negatively impact sperm quality and the success of ARTs.
In the future, the integration of multi-omics approaches that combine metagenomics, transcriptomics, and metabolomics data will provide a more functional understanding of the impact of the microbiome on male fertility.1,7 The development of next-generation probiotics, prebiotics, and other microbiome-targeted therapies, combined with the surge in machine learning and artificial intelligence (AI) models, may also provide important insights needed to develop more effective interventions for male infertility.7,11
References
- Corral-Vázquez, C., Blanco, J., Sarrate, Z., & Anton, E. (2024). Unraveling the Intricacies of the Seminal Microbiome and Its Impact on Human Fertility. Biology 13(3); 150. DOI:10.3390/biology13030150, https://www.mdpi.com/2079-7737/13/3/150.
- Liu, J., Qin, Y., Liu, H., et al. (2025). Global, regional, and national burden of female infertility and trends from 1990 to 2021 with projections to 2050 based on the GBD 2021 analysis. Scientific Reports 15(1). DOI:10.1038/s41598-025-01498-x, https://www.nature.com/articles/s41598-025-01498-x.
- Chatzokou, D., Tsarna, E., Davouti, E., et al. (2025). Semen Microbiome, Male Infertility, and Reproductive Health. International Journal of Molecular Sciences 26(4); 1446. DOI:10.3390/ijms26041446, https://www.mdpi.com/1422-0067/26/4/1446.
- Osadchiy, V., Mills, J. N., Mayer, E. A., & Eleswarapu, S. V. (2020). The Seminal Microbiome and Male Factor Infertility. Current Sexual Health Reports 12(3); 202-207. DOI:10.1007/s11930-020-00273-5, https://link.springer.com/article/10.1007/s11930-020-00273-5.
- Mowla, S., Farahani, L., Tharakan, T., et al. (2025). Characterisation and comparison of semen microbiota and bacterial load in men with infertility, recurrent miscarriage, or proven fertility. eLife. DOI:10.7554/elife.96090.3, https://elifesciences.org/reviewed-preprints/96090.
- Lv, S., Huang, J., Luo, Y., et al. (2024). Gut microbiota is involved in male reproductive function: a review. Frontiers in Microbiology 15. DOI:10.3389/fmicb.2024.1371667, https://www.frontiersin.org/articles/10.3389/fmicb.2024.1371667.
- Kaltsas, A., Giannakodimos, I., Markou, E., et al. (2025). The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility. International Journal of Molecular Sciences 26(13); 6211. DOI:10.3390/ijms26136211, https://www.mdpi.com/1422-0067/26/13/6211.
- Lindhof, H. & Homey, B. (2025). Ureaplasma parvum impaired semen quality improves after doxycycline treatment in selected patients: a cohort study. Basic and Clinical Andrology 35(1). DOI:10.1186/s12610-025-00284-z, https://basicandclinicalandrology.biomedcentral.com/articles/10.1186/s12610-025-00284-z.
- Trigg, N. A., Zhou, S. K., Harris, J. C., et al. (2025). A lack of commensal microbiota influences the male reproductive tract intergenerationally in mice. Reproduction 169(4). DOI:10.1530/rep-24-0204, https://rep.bioscientifica.com/view/journals/rep/169/4/REP-24-0204.xml
- Okwelogu, S. I., Ikechebelu, J. I., Agbakoba, N. R., & Anukam, K. C. (2021). Microbiome Compositions From Infertile Couples Seeking In Vitro Fertilization, Using 16S rRNA Gene Sequencing Methods: Any Correlation to Clinical Outcomes? Frontiers in Cellular and Infection Microbiology 11. DOI:10.3389/fcimb.2021.709372, https://www.frontiersin.org/articles/10.3389/fcimb.2021.709372.
- Oliveira, L., Costa, E. C., Martins, F. D. G., et al. (2024). Probiotics supplementation in the treatment of male infertility: A Systematic Review. JBRA Assisted Reproduction. DOI:10.5935/1518-0557.20240013, https://pmc.ncbi.nlm.nih.gov/articles/PMC11152433/
- Valcarce, D. G., Genovés, S., Riesco, M. F., Martorell, P., Herráez, M. P., Ramón, D., & Robles, V. (2017). Probiotic administration improves sperm quality in asthenozoospermic human donors. Beneficial Microbes 8(2):193–206. DOI:10.3920/BM2016.0122, https://brill.com/view/journals/bm/8/2/article-p193_6.xml?srsltid=AfmBOoqPxRrmWlBzmPTzeWyYml_TDSluZ0P7lmzpE4e_hbwYOkRmtXep
- Maretti, C., & Cavallini, G. (2017). The association of a probiotic with a prebiotic (Flortec) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology 5(3):439–444. DOI:10.1111/andr.12336, https://onlinelibrary.wiley.com/doi/10.1111/andr.12336
- Helli, B., Kavianpour, M., Ghaedi, E., Dadfar, M., & Haghighian, H. K. (2022). Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men: A randomized trial. Human Fertility (Camb) 25(4):499–507. DOI:10.1080/14647273.2020.1824080, https://www.tandfonline.com/doi/full/10.1080/14647273.2020.1824080
- Abbasi, B., Abbasi, H., & Niroumand, H. (2021). Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. International Journal of Reproductive Biomedicine 19(3):235–244. DOI:10.18502/ijrm.v19i3.8571, https://pmc.ncbi.nlm.nih.gov/articles/PMC8023005/
Further Reading
Last Updated: Sep 21, 2025