Introduction
What is Prime Editing?
How Does Prime Editing Work?
Advantages of Prime Editing
Potential Applications in Health and Medicine
Current Status of Clinical Research
Challenges and Ethical Considerations
Conclusions
References
Further Reading
Discover how prime editing is redefining the future of medicine by offering highly precise, safe, and versatile DNA corrections, bringing hope for more effective treatments for genetic diseases while raising important scientific and ethical questions.
 Image Credit: Anusorn Nakdee / Shutterstock.com
Image Credit: Anusorn Nakdee / Shutterstock.com
Introduction
What if we could rewrite the genetic code of a single cell with surgical precision? Prime editing, the next leap in genome engineering, enables highly accurate DNA corrections without damaging the genetic code, paving the way for safer and more effective therapies for genetic disorders.
As technology evolves, prime editing shows promise in becoming a cornerstone of personalized medicine. This article explores the fundamentals of prime editing, its differences from other gene editing technologies, the underlying mechanisms, potential medical applications, as well as the scientific and ethical challenges that lie ahead.
What is Prime Editing?
Introduced in 2019 by a team of researchers from the Broad Institute at the Massachusetts Institute of Technology and Harvard University, prime editing builds upon clustered regularly interspaced short palindromic repeats (CRISPR), redefining therapeutic approaches to various genetic disorders.1
Prime editing is a next-generation gene-editing technique designed to correct, insert, or delete specific DNA sequences without causing double-stranded breaks or relying on donor DNA templates. As compared to earlier genome editing methods such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR-CRISPR-associated protein 9 (Cas9), prime editing offers a more precise and flexible alternative with fewer off-target effects.3,4
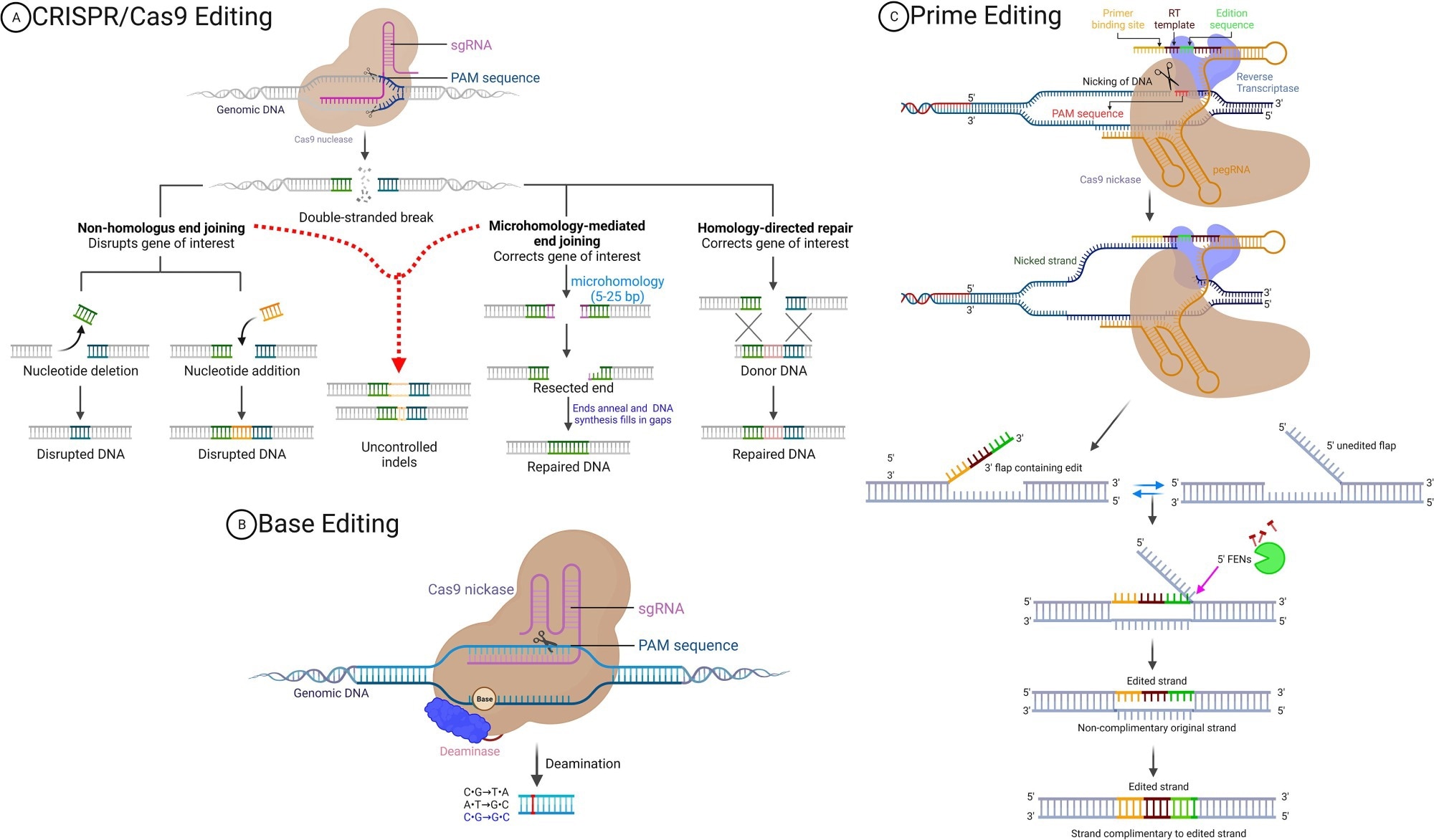
Conventional CRISPR-Cas9 systems edit DNA by creating double-stranded breaks at targeted genomic locations, followed by cellular repair through either non-homologous end joining or homology-directed repair. While effective, this process can result in unintended DNA insertions or deletions, also known as indels, and is primarily limited to dividing cells.5
Comparatively, prime editing relies on Cas9 nickase, a modified CRISPR-Cas9 system, fused to a reverse transcriptase enzyme capable of identifying and subsequently replacing specific DNA sequences with higher precision and lower risk.5 Since its introduction in 2019 by David Liu and colleagues, prime editing has rapidly become a focus of intensive research, with several improvements that have enhanced its efficiency and applicability in therapeutic settings.6
 Graphical description of the Cas9-mediated genome editing technologies.4
Graphical description of the Cas9-mediated genome editing technologies.4
How Does Prime Editing Work?
Prime editors (PEs) consist of a three-component system including a Cas9 nickase, which is conjugated to an engineered reverse transcriptase paired with a prime editing guide ribonucleic acid (pegRNA). Initially, pegRNA will bind to the target DNA sequence through complementary base pairing, following which the Cas9 nickase nicks the non-target strand.
pegRNA serves the dual purpose of guiding the PE protein to the targeted locus and encoding the desired edit.”
The pegRNA subsequently uses the reverse transcription template to synthesize the desired DNA sequence at the break site. Ligation and DNA mismatch repair (MMR) are then initiated to copy information from the editing strand to the unedited strand, which results in the desired modification to the genome.3,6
"Prime Editing" Revolutionizing Genetic Engineering
Advantages of Prime Editing
Prime editing offers numerous advantages as compared to earlier genome editing tools.
The versatility of prime editing, for example, enables all 12 possible base-to-base conversions and supports small insertions and deletions, thus offering immense flexibility for precise genomic modifications. Prime editing is also highly precise, which significantly reduces the risk of unintended mutations and off-target effects as compared to CRISPR-Cas9, thereby making it a safer alternative for therapeutic applications.7
Prime editing can function in both dividing and non-dividing cells, thereby broadening its application in tissues such as neurons and muscle cells that were previously challenging to edit. This technology also eliminates the need for double-strand breaks or donor templates, thereby minimizing cellular stress and enhancing editing outcomes.5
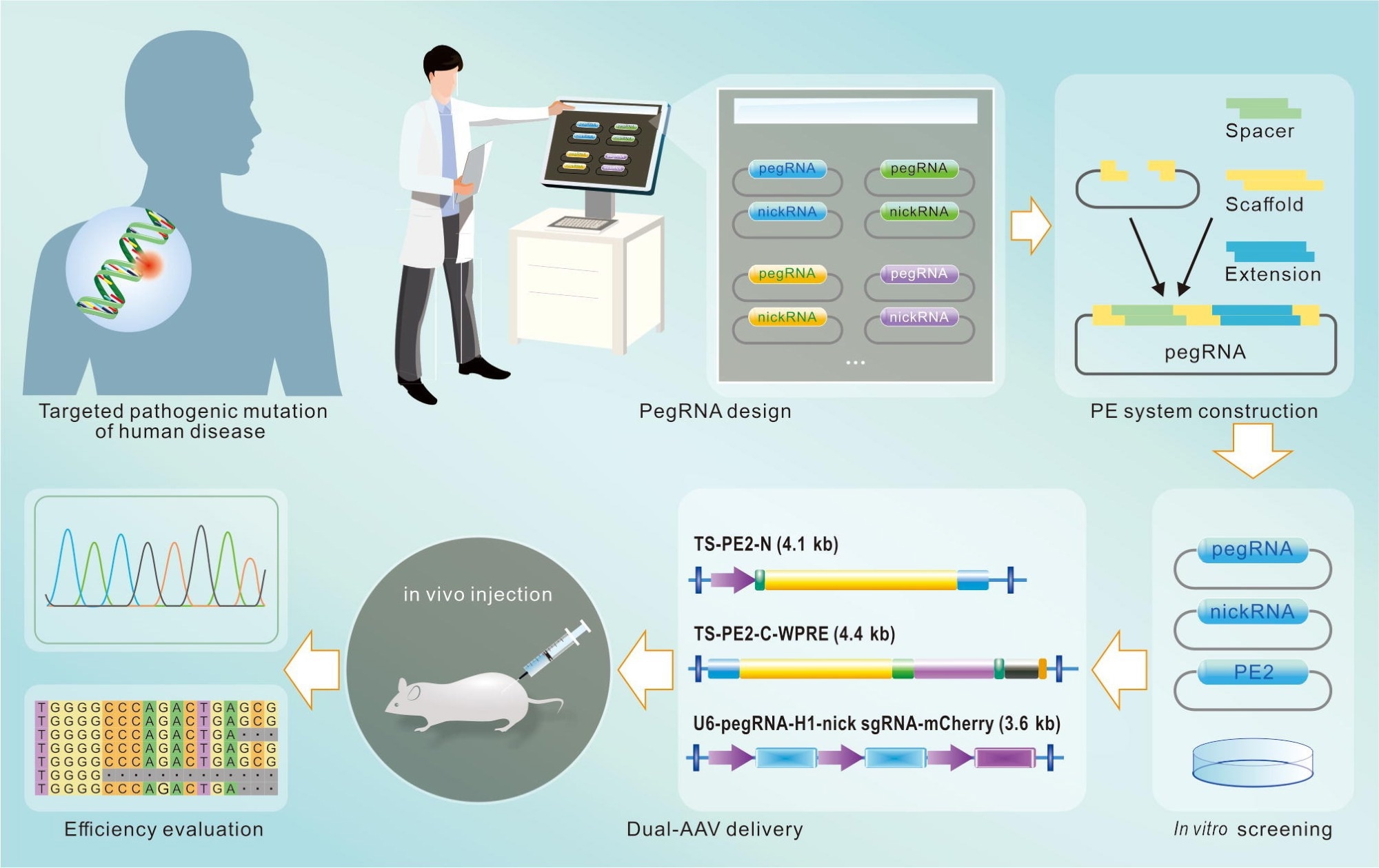 The flowchart of PE design and application. Prime editing is used to treat genetic diseases with specific pathogenic mutations. Some websites may help to design a variety of candidate pegRNAs and nsgRNAs, which are then constructed by means of the golden gate assembly technique. Subsequently, these plasmids are applied for in vitro screening to choose the pegRNA and nsgRNA with the best performance. These well-performed RNAs are then constructed into a dual-AAV system. After being injected into the mouse model, the editing efficiency and off-target events of prime editing are finally evaluated.
The flowchart of PE design and application. Prime editing is used to treat genetic diseases with specific pathogenic mutations. Some websites may help to design a variety of candidate pegRNAs and nsgRNAs, which are then constructed by means of the golden gate assembly technique. Subsequently, these plasmids are applied for in vitro screening to choose the pegRNA and nsgRNA with the best performance. These well-performed RNAs are then constructed into a dual-AAV system. After being injected into the mouse model, the editing efficiency and off-target events of prime editing are finally evaluated.
Potential Applications in Health and Medicine
Prime editing is currently being explored as a novel approach for the treatment of genetic diseases, cancer, and infectious diseases.
This efficient tool can correct up to 89% of previously discovered genetic variants that are associated with human diseases.”
Monogenic Diseases
Prime editing has been widely studied in both in vitro and in vivo preclinical models to correct genetic mutations responsible for Duchenne muscular dystrophy, Leber congenital amaurosis, tyrosinemia, α1-Antitrypsin deficiency (AATD), and phenylketonuria. Although numerous prime editors (PEs) have been investigated and provided promising results, additional studies using higher animal models are warranted before this technology can be evaluated in human subjects.3
Cancer research
Chemotherapy, radiation, and surgery are the primary therapies used for the treatment of cancer, in addition to hormonal and targeted immunotherapies when applicable. However, these approaches are associated with numerous unwanted and critical side effects, which warrant the development of safe and more targeted strategies.
Various genetic mutations have been implicated in cancer as the driving cause of non-cancerous cells transforming into cancer cells due to altered protein expression. Gene editing technologies like CRISPR Cas9 and prime editing have been widely studied for their potential to correct these malignant mutations, as well as induce cancer-related mutations in preclinical models used to investigate novel therapeutics.
Infectious diseases
There is growing interest in using prime editing to offer resistance to viral infections or remove integrated viral genomes from host DNA, thereby preventing the virus from being reactivated. This could provide permanent immunity or viral elimination.4
Preventive genomics
Making precise changes in the genome raises the possibility of using prime editing in germ cells or embryos to create changes that could be inherited. However, these applications remain ethically contentious and tightly regulated.10
Current Status of Clinical Research
Most prime editing applications are preclinical and, as a result, have been limited to in vitro and in vivo models. However, ex vivo editing of patient cells followed by transplantation has provided substantial hope for successful future clinical translation.8
Challenges and Ethical Considerations
Several scientific, technical, and ethical concerns must be addressed before prime editing becomes standard practice. The large size of PEs makes delivery through viral vectors difficult, which has led researchers to investigate the potential utility of non-viral platforms and optimized vectors for prime editing applications.5
Editing efficiency also varies significantly depending on the location of the gene and the type of cell. To overcome these challenges, researchers are actively developing strategies to improve pegRNA design, reverse transcriptase activity, and the cellular repair environment.1
The misuse of genome editing for non-therapeutic purposes and the enhancement of designer traits is a primary ethical concern. To address these issues, regulatory bodies such as the United States Food and Drug Administration (FDA) are developing frameworks for the responsible use of prime editing technology.10
Conclusions
Prime editing represents a powerful tool for genetic engineering.
Further research is needed to continue advancing gene editing technologies, focusing on increasing efficiency, improving DNA delivery, and enhancing safety. An interdisciplinary approach and continued public engagement are also crucial to ensure that prime editing is used safely and ethically.
References
- Zhao, Z., Shang, P., Mohanraju, P., & Geijsen, N. (2023). Prime editing: advances and therapeutic applications. Trends in Biotechnology, 41(8), 1000–1012. DOI:10.1016/j.tibtech.2023.03.004, https://www.sciencedirect.com/science/article/pii/S0167779923000859
- Anzalone, A. V., Randolph, P. B., Davis, J. R., Sousa, A. A., Koblan, L. W., Levy, J. M., Chen, P. J., Wilson, C., Newby, G. A., Raguram, A., & Liu, D. R. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 576(7785), 149–157. DOI:10.1038/s41586-019-1711-4, https://www.nature.com/articles/s41586-019-1711-4
- Fu, Y., He, X., Gao, X. D., Li, F., Ge, S., Yang, Z., & Fan, X. (2023). Prime editing: current advances and therapeutic opportunities in human diseases. Science Bulletin, 68(24), 3278–3291. DOI:10.1016/j.scib.2023.11.015, https://www.sciencedirect.com/science/article/pii/S2095927323007697
- Hosseini, S. Y., Mallick, R., Mäkinen, P., & Ylä-Herttuala, S. (2024). Insights into Prime Editing Technology: A Deep Dive into Fundamentals, Potentials, and Challenges. Human Gene Therapy, 35(17-18), 649–668. DOI:10.1089/hum.2024.043, https://www.liebertpub.com/doi/10.1089/hum.2024.043
- Li, M., Lin, Y., Cheng, Q., & Wei, T. (2025). Prime Editing: A Revolutionary Technology for Precise Treatment of Genetic Disorders. Cell Proliferation, 58(4), e13808. DOI:10.1111/cpr.13808, https://onlinelibrary.wiley.com/doi/full/10.1111/cpr.13808
- Scholefield, J., & Harrison, P. T. (2021). Prime Editing - An Update on the Field. Gene Therapy, 28(7-8), 396–401. DOI:10.1038/s41434-021-00263-9, https://www.nature.com/articles/s41434-021-00263-9
- Gao, P., Lyu, Q., Ghanam, A. R., Lazzarotto, C. R., Newby, G. A., Zhang, W., Choi, M., Slivano, O. J., Holden, K., Walker, J. A., 2nd, Kadina, A. P., Munroe, R. J., Abratte, C. M., Schimenti, J. C., Liu, D. R., Tsai, S. Q., Long, X., & Miano, J. M. (2021). Prime editing in mice reveals the essentiality of a single base in driving tissue-specific gene expression. Genome Biology, 22(1), 83. DOI:10.1186/s13059-021-02304-3, https://genomebiology.biomedcentral.com/articles/10.1186/s13059-021-02304-3
- Everette, K.A., Newby, G.A., Levine, R.M. et al. Ex vivo prime editing of patient haematopoietic stem cells rescues sickle-cell disease phenotypes after engraftment in mice. Nature Biomedical Engineering 7, 616–628 (2023). DOI:10.1038/s41551-023-01026-0, https://www.nature.com/articles/s41551-023-01026-0
- Sousa, A. A., Hemez, C., Lei, L., Traore, S., Kulhankova, K., Newby, G. A., Doman, J. L., Oye, K., Pandey, S., Karp, P. H., McCray, P. B., Jr, & Liu, D. R. (2025). Systematic optimization of prime editing for the efficient functional correction of CFTR F508del in human airway epithelial cells. Nature Biomedical Engineering, 9(1), 7–21. DOI:10.1038/s41551-024-01233-3, https://www.nature.com/articles/s41551-024-01233-3
- Rothschild J. (2020). Ethical considerations of gene editing and genetic selection. Journal of General and Family Medicine, 21(3), 37–47. DOI:10.1002/jgf2.321, https://onlinelibrary.wiley.com/doi/full/10.1002/jgf2.321
Further Reading
Last Updated: Jun 3, 2025