This article and associated images are based on a poster originally authored by Oliver Carney, Louis Hodgson, and Carl Peters, and presented at ELRIG Drug Discovery 2025 in affiliation with BMG LABTECH and Albert Einstein College of Medicine.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Small G-proteins or small GTPases are a family of nearly 200 proteins in humans that can bind and hydrolyze guanosine triphosphate (GTP). They are considered active when they are bound to GTP and inactive when bound to GDP.
They signal to a wide variety of effector proteins and are involved in regulating cellular processes, such as growth and cell movement. Dysregulated small G-protein function has been associated with many diseases, including cancer.1
The best characterized small G-proteins belong to the Ras superfamily, which is further subdivided into five groups, including the Rho GTPase family. Rho GTPase family members play a crucial role in the dynamic regulation of the cell cytoskeleton, contributing to cell movement and cancer progression.2
Foerster’s Resonance Energy Transfer (FRET) biosensors have proved to be a valuable tool in the study of Rho GTPases.3 The family members are known to act together, so being able to observe their actions simultaneously is vital to our understanding.4
Here, the research team describes a FRET biosensor for Rac1 that utilizes near-infrared (NIR) fluorescent proteins, allowing for spectral resolution from existing biosensors based on the blue/green/yellow spectral range, while also being less susceptible to autofluorescence issues commonly observed in cell-based samples.3
The spectral scanning capabilities of the CLARIOstar Plus proved useful in monitoring the function of these biosensors in a multiplexing approach.
Assay principle
Figure 1 shows a diagram of the NIR FRET Rac1 biosensor.3 It takes advantage of the molecular switch nature of small G-proteins. In the active GTP-bound state, binding of the p-21 binding domain (PBD), derived from p21-activated kinase 1, to Rac1-GTP occurs very easily.
The biosensor incorporates a second PBD that has GTPase-binding deficient mutations (Figure 1; depicted in red). This serves as an auto-inhibitory motif to limit FRET-competent interactions to the presence of active GTP-bound Rac1.
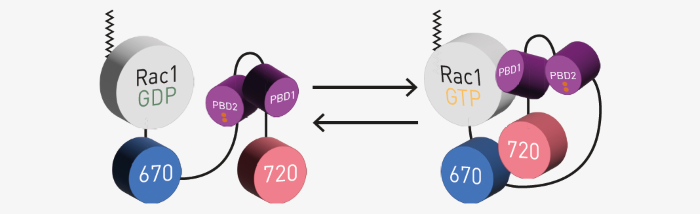
Fig 1. NIR FRET Rac1 biosensor assay principle. Image Credit: Image courtesy of Oliver Carney et al., in partnership with ELRIG (UK) Ltd.
The biosensors use miRFP670 and miRFP720 as a FRET pair attached to the C-terminus of Rac1. Two PBDs provide appropriately tuned interaction with Rac1 (adapted from Shcherbachova et al.).3
Materials and methods
- 12-well, clear, TC-treated microplates (Costar)
- CLARIOstarPlus with red extended PMT
- Other chemicals and reagents were obtained from commercial sources
Experimental procedure
HEK293 cells were transfected with plasmids carrying the indicated Rac1 WT or Rac1 mutant biosensors. A total of 400 ng of biosensor plasmid was used in 100 μL OptiMEM solution (all DNA amounts listed here are optimized for the six-well plate format. For the 12-well plate format, 50 % of this mixture was then used to set up technical duplicates).
Where indicated, cells were additionally transfected with modifying proteins; 400 ng of these plasmids were used except for 4x GDI (1600 ng). Empty plasmid control DNA was added to a total of 2 μg of DNA as needed.
Transfections were performed using the PEI reagent, and the transfection mixture volume, cell culture media volume per well, and cell seeding densities were appropriately optimized for the 12-well plate format.5 Adherent cells were read in clear L15 media with 10 % FBS at room temperature.
Instrument settings. Source: ELRIG (UK) Ltd.
| |
|
|
| Optic settings |
Fluorescence Intensity, spectrum |
| Excitation wavelength (nm) |
591-52 |
| Emission wavelengths (nm) |
640-15 to 802-15 |
| Gain |
2600 |
| Focal height |
4.8 |
| General settings |
Flashes |
60 |
| Settling time (seconds) |
0.5 |
Results and discussion
Spectral data were blank corrected using spectra from HEK293 cells transfected with a control empty plasmid. The data was then scaled using the RFU at 676 nm as 100 %. Representative data are shown in Figure 2.
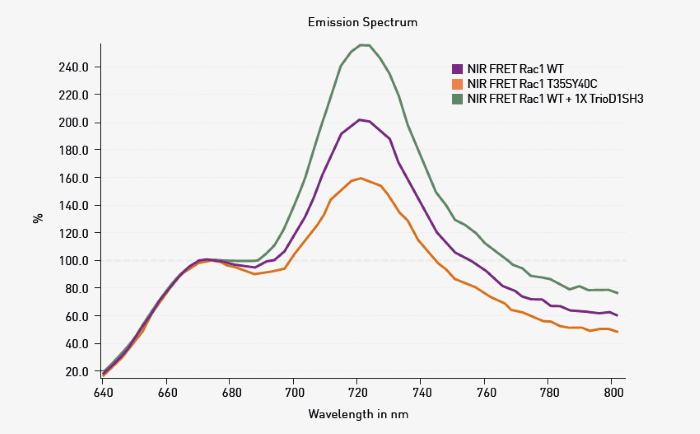
Fig 2. Spectral scanning of NIR FRET biosensors. HEK293 cells were transfected as indicated. Spectral scanning shows alteration in FRET based on changes in the peak at 721 nm. Image Credit: Image courtesy of Oliver Carney et al., in partnership with ELRIG (UK) Ltd.
As expected for the conditions used, WT Rac1 exhibited FRET signal transfer due to Rac1 activation, resulting in a signal increase at 721 nm. The extent of FRET was decreased in T35S-Y40C Rac1. Since this mutant should not bind the PBD built into the sensor, the response agrees with the expectations.
Finally, the expected increase in FRET could be seen upon the addition of an activator protein fragment (TrioD1SH3), which was co-transfected with WT NIR-Rac1. The experiments shown in Figure 2 were repeated, and additional mutations and co-transfections were tested (Figure 3).
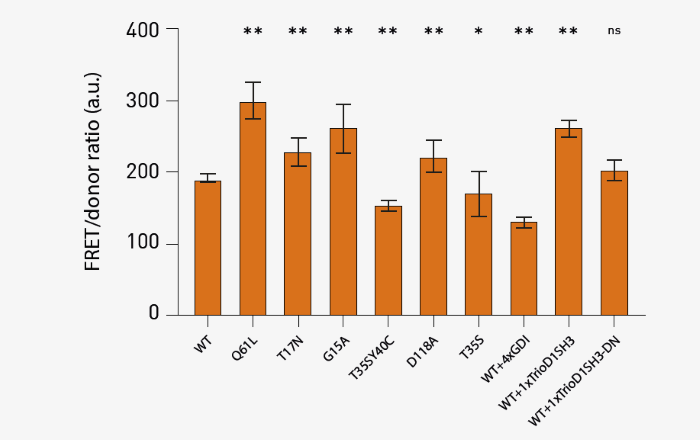
Fig 3. FRET responses determined from spectral scan results. Results from 10 total technical replicates spanning 3 different biological replicates are shown. Average +/- SD (n=3; ** p<0.01; * p<0.05; “ns” not significant, paired Student t-test, all compared against the WT condition) of the value at 721 nm based on spectral scan measurements. Image Credit: Image courtesy of Oliver Carney et al., in partnership with ELRIG (UK) Ltd.
The results in Figure 3 were in line with the expectations for mutations and co-transfections. Of note, the Q61L exhibited a significant increase in FRET, which agrees with this being the strongest activating mutation of Rac1. Three dominant negative mutants (T17N, G15A, and D118A) exhibited a moderate increase in FRET signal, suggesting that stable interaction with upstream activators enhances FRET.
Effector binding mutations (T35SY40C and T35S) showed reduced FRET. In addition, co-transfection of GDI at four times the excess, a negative inhibitor protein that binds and deactivates GTPase, significantly reduced WT FRET. The addition of the activator fragment of Rac1 (TrioD1SH3) to the WT biosensor increased FRET signal, while the same fragment - but with catalytically deactivating mutations (TrioD1SH3-DN) - did not affect the WT FRET.
Conclusion
The research team demonstrates the validation of the NIR FRET Rac1 biosensors as a viable tool for detecting changes in Rac1 activation. Mutations of Rac1 or co-expression of modifying proteins had the expected effect on FRET as assessed by spectral scanning using the CLARIOstarPlus.
This was achieved using the Linear Variable Filter (LVF) monochromator that offers flexibility and filter-like sensitivity. These biosensors will be useful tools in furthering the understanding of complex small G-protein signaling.
References
- Yin, G., et al (2023). Targeting small GTPases: emerging grasps on previously untamable targets, pioneered by KRAS. Signal Transduction and Targeted Therapy, (online) 8(1). https://doi.org/10.1038/s41392-023-01441-4.
- Vega, F.M. and Ridley, A.J. (2008). Rho GTPases in cancer cell biology. FEBS Letters, 582(14), pp.2093–2101. https://doi.org/10.1016/j.febslet.2008.04.039.
- Shcherbakova, D.M., et al. (2018). Direct multiplex imaging and optogenetics of Rho GTPases enabled by near-infrared FRET. Nature Chemical Biology, 14(6), pp.591–600. https://doi.org/10.1038/s41589-018-0044-1.
- Machacek, M., et al. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature, (online) 461(7260), pp.99–103. https://doi.org/10.1038/nature08242.
- Ehrhardt, C., et al. (2006). Polyethylenimine, a cost-effective transfection reagent. Signal Transduction, 6(3), pp.179–184. https://doi.org/10.1002/sita.200500073.
About BMG LABECH
BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025