This article is based on a poster originally authored by R. Pinto-Lopes, M. Kerr, B. Boyle, G. PJMullan, and K Thompson.
Intestinal methanogen overgrowth (IMO) is characterized by an excessive presence of methane-producing archaea in the gastrointestinal tract and is associated with symptoms such as bloating, abdominal pain, and constipation.1
Hydrogen and methane breath tests (HMBTs), both conducted in-clinic and as send-out tests, are commonly utilized for diagnosis. These tests typically involve a challenge procedure, wherein a glucose or lactulose substrate is ingested, with IMO diagnosed based on a breath methane peak exceeding 10 ppm at any point during the assessment.2
However, these evaluations necessitate patient preparation and are constrained by the limited number of breath samples collected during the testing process.
The study discussed here evaluates the OMED Health® Breath Analyzer for its capacity to accurately measure breath methane and hydrogen.
Preliminary data illustrating its potential for diagnosing and longitudinally monitoring treatment responses in IMO within a real-world context is presented.
Method
OMED Health® Breath Analyzer
The OMED Health® Breath Analyzer is a handheld device designed to measure hydrogen and methane concentrations in exhaled breath in real time.
Utilizing metal oxide sensor (MOS) technology, along with diffusion and PTFE membranes, the device minimizes interference from volatile organic compounds (VOCs) and condensation, thereby ensuring stable and reliable measurements (Figure 1).
Data is recorded and displayed through a companion smartphone application (Figure 2).
Breath collection and analysis
Hydrogen and methane concentrations from participant breath samples were measured using two methods: a gold-standard in-clinic HMBT instrument (Gastrogenius™ Breath Monitor) and the OMED Health® Breath Analyzer.3
Each participant provided three breath samples, spaced three minutes apart, in the following order: in-clinic instrument, OMED Health® device, in-clinic instrument. To account for variations in absolute gas concentrations between samples, the two readings from the in-clinic instrument were averaged (AVG HMBT) and utilized for comparison.
Diagnostic classification
A subset of participants was evaluated for IMO (n = 24) and small intestinal bacterial overgrowth (SIBO; n = 14) using the in-clinic HMBT instrument and the OMED Health® device. IMO was defined as any methane reading exceeding 10 ppm during the test, while SIBO was defined as a greater than 10 ppm increase in hydrogen within 65 minutes of lactulose ingestion (EU criterion).
Case study
This case study presents a patient diagnosed with IMO, initially treated with herbal antimicrobials followed by antibiotic therapies. Breath data was collected using the OMED Health® Breath Analyzer to monitor treatment responses over time.
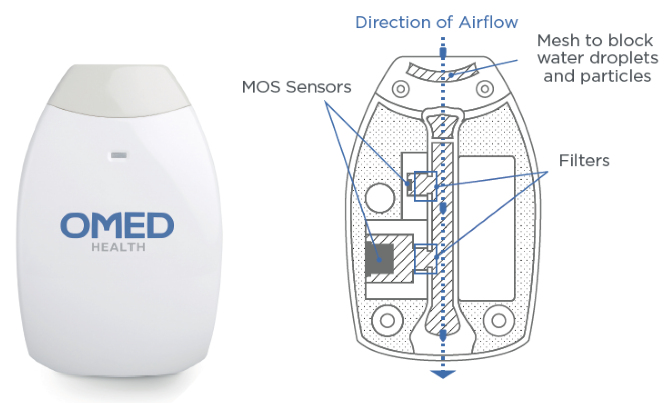
Figure 1. Schematic of the OMED health® Breath Analyser, showing the (patent pending) airflow path, filter design, and MOS technology. Image Credit: Owlstone Medical Ltd

Figure 2. The OMED Health Breath Analyzer and companion mobile app provides a direct, real-time readout of breath hydrogen and methane levels. The companion mobile app also supports the breath sampling protocol, records breath measurements and facilitates the logging of food/drink and the tracking of symptoms and lifestyle. Image Credit: Owlstone Medical Ltd
Results
Device comparison
A total of 243 breath samples were collected from 54 participants, with complete data available for hydrogen and methane measurements from both devices. This data facilitated a direct comparison of hydrogen and methane concentrations between the OMED Health® Breath Analyzer and the gold-standard in-clinic HMBT instrument.
Results demonstrated comparable error distributions for both gases. A slight positive bias was noted in OMED hydrogen readings relative to the in-clinic HMBT (Figure 3A); however, this did not impact the diagnostic accuracy of the device.
Diagnostic concordance
Diagnostic results for IMO and SIBO derived from the OMED Health® Breath Analyzer were compared against those from the in-clinic HMBT instrument, revealing greater than 90 % concordance.
The confusion matrix (Figure 3B) illustrates the number and proportion of matched and unmatched outcomes. High concordance was observed, with only one false positive for IMO and one false negative for SIBO identified.
Case study: IMO monitoring with OMED
Patient A was diagnosed with Intestinal Methanogen Overgrowth (IMO) on May 7, 2024, presenting a daily average methane level of 23.6 ± 1.79 ppm. Following the initiation of herbal antimicrobial therapy on June 13, methane levels decreased from 35.7 ppm to 2.64 ppm over a two-week period.
Subsequent continuous, non-fasting monitoring revealed a rebound in methane levels, resulting in a second IMO-positive diagnosis on July 16, with an average of 24.3 ± 3.79 ppm.
A follow-up course of antibiotics successfully reduced methane levels from 32.8 ppm to 3.56 ppm within two weeks, after which the patient reported a negative result for IMO. By September, methane levels remained consistently low at 3.07 ± 0.94 ppm. These findings are summarized in Figures 3C and 3D.
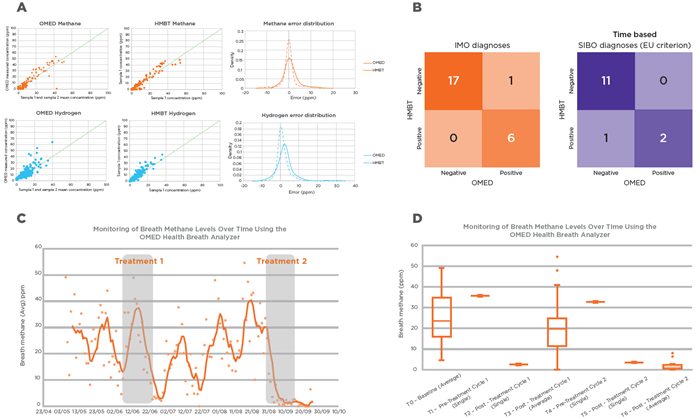
Figure 3. A) Comparison of average hydrogen and methane values from the HMBT (mean of two consecutive samples) with corresponding OMED Health® Breath Analyzer measurements, including error distributions. B) Diagnostic performance of the OMED Health® Breath Analyzer compared to gold-standard HMBT testing for IMO and SIBO. High concordance was observed, with >90 % accuracy for both diagnoses (per EU criteria). C) Daily average breath methane levels in Patient A before and after treatment 1, with recurrence and resolution following treatment 2. D) Mean breath methane levels in Patient A pre- and post-treatment 1 and 2, showing treatment response and sustained reduction post-treatment 2. Image Credit: Owlstone Medical Ltd
Conclusions
Longitudinal sampling is increasingly recognized for its ability to facilitate real-time analysis and establish personalized baselines, thereby supporting a transition from static to continuous monitoring in clinical care.
This approach is exemplified by the evolution from fingerstick blood sampling to continuous glucose monitoring in diabetes management. When applied to breath analysis, the OMED Health® Breath Analyzer enables efficient detection and real-time monitoring of treatment responses in gastrointestinal conditions such as IMO.
In the study discussed here, the device demonstrated over 90 % diagnostic concordance with the gold standard in-clinic Hydrogen Methane Breath Test (HMBT) for both IMO and Small Intestinal Bacterial Overgrowth (SIBO), underscoring its potential clinical utility.
Beyond IMO, breath methane monitoring shows promise in broader contexts, including gut motility, inflammatory regulation, and metabolic health.4
These findings emphasize the value of at-home breath testing conducted under clinical guidance to ensure accurate interpretation and optimized treatment outcomes.
References and further reading
- Mohajeri, M.H., et al. (2018). The role of the microbiome for human health: from basic science to clinical applications. European Journal of Nutrition, (online) 57(S1), pp.1–14. https://doi.org/10.1007/s00394-018-1703-4.
- Rezaie, A., et al. (2017). Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. The American Journal of Gastroenterology, (online) 112(5), pp.775–784. https://doi.org/10.1038/ajg.2017.46.
- Laborie / Medical Measurement Systems B.V. (n.d.). Netherlands.
- Kerr, M., et al. (2024). Beyond the Gut: Unveiling Methane’s Role in Broader Physiological Systems. (online) https://doi.org/10.20944/preprints202411.0391.v1.
Acknowledgements
Produced form material originally authored by R. Pinto-Lopes, M. Kerr and B. Boyle from Owlstone Medical Ltd, and G. PJ Mullan and K Thompson from humanpeople.
About Owlstone Medical Ltd
Owlstone Medical is developing a breathalyzer with a focus on non-invasive diagnostics for cancer, inflammatory disease and infectious disease, the company aims to save 100,000 lives and $1.5 B in healthcare costs.
The company’s Breath Biopsy® platform has introduced a new diagnostic modality making it possible to discover novel non-invasive biomarkers in breath using a platform with the potential to transition to point-of-care. The award winning ReCIVA Breath Sampler ensures reliable collection of breath samples.
Breath Biopsy is supporting research into early detection and precision medicine with applications in cancer and a wide range of other medical conditions. Highly sensitive and selective, these tests allow for early diagnosis when treatments are more effective and more lives can be saved.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Sep 16, 2025