Please can you introduce yourself and tell us about your role at Owlstone Medical?
My name is Mariana Leal, and I am a Lead Translational Scientist – Team Lead at Owlstone Medical. Before I joined Owlstone Medical, I worked for a long time in academia, mainly in the oncology field and then in a drug discovery biotech. My work involved biomarker discovery and also understanding the mechanism of action and resistance to several anticancer therapies using clinical samples and pre-clinical models.
I joined Owlstone because the idea of creating a non-invasive test is a key motivator for any translational scientist working with challenging diseases such as cancers. I was intrigued by the technology that Owlstone Medical was developing and the possibility of addressing the challenge of an early cancer diagnosis as part of a multidisciplinary team.
One of my key roles in Owlstone Medical involves bringing together the knowledge of biomarker discovery and cancer metabolism to develop probes that will be used as part of minimally invasive or non-invasive tests to detect lung cancer.
Owlstone Medical is a global leader in breath biopsy for early detection and precision medicine. Can you tell us more about Owlstone Medical and how the breath biopsies are a useful tool for investigating biomarkers for the early detection of diseases?
Owlstone Medical aims to save 100,000 lives and $1.5 billion in healthcare costs through Breath Biopsy. Our analysis is focused on volatile organic compounds (VOCs) present in the exhaled breath. These VOCs are the product of metabolic processes within a person’s cells and tissues, within their microbiome, and from their response to environmental exposures. Since VOC production is linked directly to metabolic activity, the compounds can be sampled quickly and noninvasively from breath, urine, or other bodily fluids. Changes in VOC levels take place at the very earliest stages of disease, so detection of the compounds can allow disease diagnosis before other physical symptoms become apparent.
Introducing Owlstone Medical - Breath Biopsy for Non-invasive Biomarkers
One particular focus area for Owlstone Medical is surrounding lung cancer. Why did you choose to particularly focus on this deadly disease, and how can your biopsy technology be utilized in this research field?
Lung cancer is one of the leading causes of cancer-related death worldwide. A strong contributor to this fact arises from the difficulty in detecting lung cancer at an early stage. Widespread public screening programs for lung cancer targeting at-risk populations represent one of the greatest opportunities to improve early detection.
However, currently, the only suitable methods (e.g., CT scanning) are a limited resource that requires specialist capabilities and are not easily accessible to the majority of the population. A breath test for lung cancer represents a non-invasive, preferable approach for screening that would be more affordable, easy to use, and accessible than current options.
We have observed an increased number of indeterminate pulmonary nodules (IPN) needing further management with the improvement of the current screening programs mainly based on CT scanning. Although the concept of IPN varies among guidelines, a nodule is considered indeterminate if the etiology is not apparent on a CT scan and is typically defined as non-calcified and solid components between 6-30mm in largest diameters.
When an IPN is identified on a CT scan, the probability of cancer is usually estimated from a clinical prediction model and defined from the management guidelines. Several blood and imaging biomarkers have recently been developed to define the probability of IPNs; however, none has yet demonstrated improvements in patient outcomes. This highlights the need for complementary approaches (including a breath biopsy) to define whether a nodule in the lung is malignant.
Despite a growing body of evidence that demonstrates the potential for breath as a means to detect illnesses, there has been limited progress in developing a breath test for cancer. As well as VOCs, exogenous volatile organic compounds (eVOCs) probes have been explored by Owlstone Medical to target various diseases. Can you tell us more about eVOCs?How can they help to tackle the limitation of the breath analysis based on volatomics?
eVOCs are compounds not normally found on breath at significant levels. However, Owsltone Medical is using these exogenous compounds to explore how the body absorbs, metabolizes, or excretes these compounds, revealing dysregulated critical pathways in different diseases.
An example of an eVOC probe under investigation for the detection of chronic liver disease is using compounds that are FDA “Generally Recognized as Safe “(GRAS) compounds, such as some food additives. These eVOCs are administered orally, undergo metabolism in the body, and are excreted via breath. This approach is exciting not only because we can amplify the signal from key metabolic pathways but also because the eVOC probe needs to be safe to be part of a breath test for diagnosis or monitoring.
The exogenous volatiles (such as the food additives) sometimes are not enough to target some disease-specific pathways; thus, we are also developing probes with more complex structures. For example, we conjugate the substrate of enzymes with a volatile tag (as presented at the AACR 2023 congress), and the release of this tag and its detection in breath is associated with the presence of the target enzyme that cleaved our probe.
Thus, eVOC probes enable the development of research tests for applications where naturally occurring VOCs alone may be insufficient and make it possible to run smaller, more focused, and potentially faster clinical trials for us and our customers.
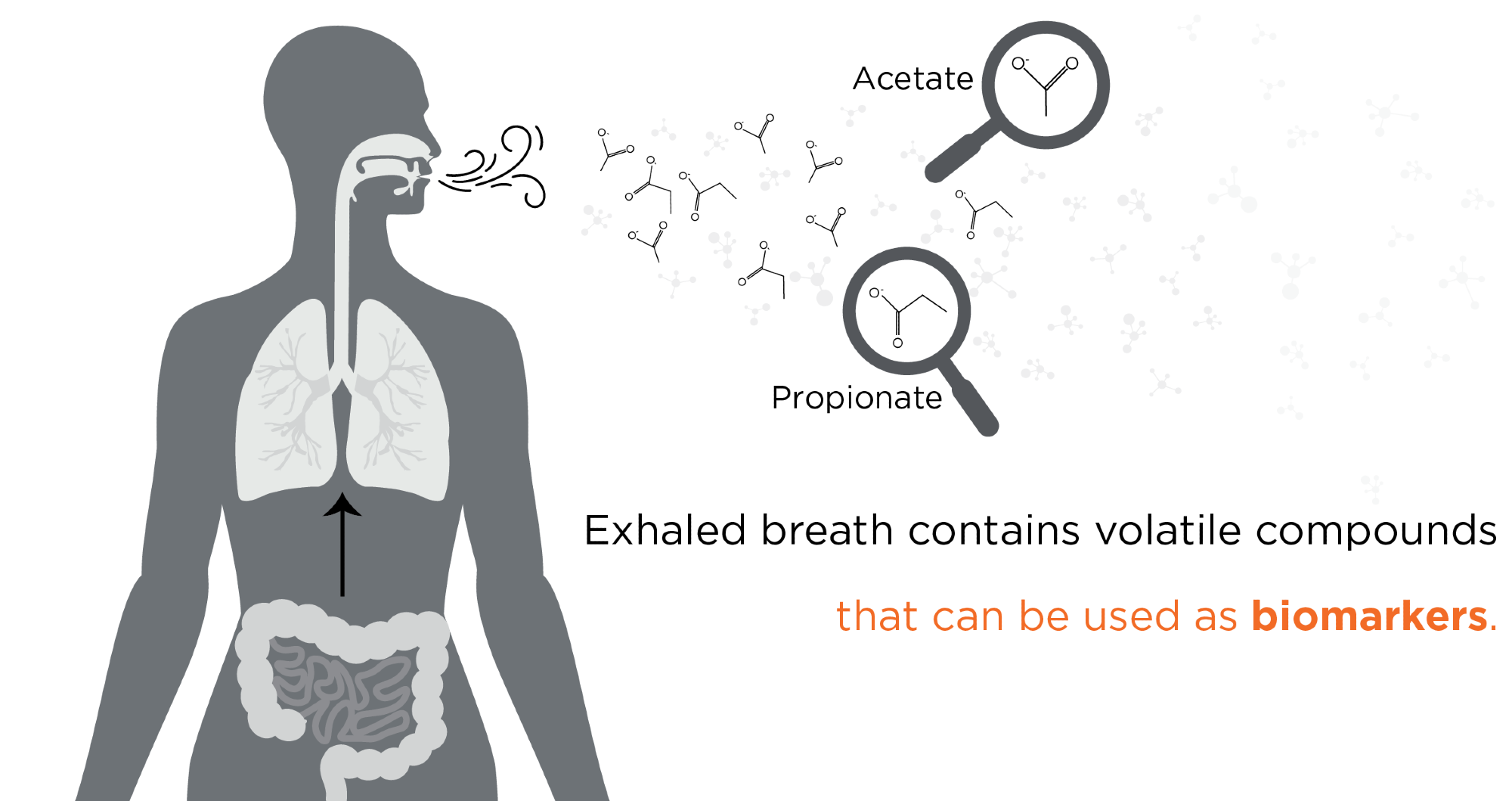
Image Credit: Owlstone Medical
At AACR, you are presenting a research poster that investigates the breath-based detection of lung cancer using exogenous volatile organic compounds (eVOCs) in humans. Can you talk us through how you conducted this research and your preliminary findings?
At AACR, we presented the results of our clinical trial Evolution, an eVOC proof-of-concept study. In the Evolution study, we evaluate whether administering a probe compound, specific to tumour-associated extracellular β-glucuronidase, produces a unique EVOC on breath of a patient with lung cancer.
We developed D5-ethyl-βD-glucuronide as a hydrophilic substrate probe that, upon hydrolysis by β-glucuronidase, releases D5-ethanol as a unique volatile reporter. We confirmed the cleavage of our probe by the target enzyme and, therefore, the detection of the deuterated labeled ethanol using our in vitro headspace system. It is important to highlight that a similar probe (same mechanism) was previously tested in in vivo cancer mouse models (Langer et al. 2019). At Poitiers University, it was previously observed that cancer-bearing animals treated with a D5-ethyl-βD-glucuronide excrete high levels of D5-ethanol more than healthy animals, and the levels of D5-ethanol observed were proportional to the tumor volume.
To establish the concept, we accessed the utility of our target probe by evaluating β-glucuronidase activity in samples from a lung cancer PDX model and the protein expression by immunohistochemistry using a large cohort of samples from human primary lung tumors, lymph node metastases, and otherwise normal lung tissue. Immunostaining of lung resection specimens showed elevated levels of extracellular β-glucuronidase in 88% of stage 1 non-small cell lung cancers, 83% of stage 2, and 95% of stage 3. For adjacent non-malignant tissue in lung cancer subjects and non-malignant lung tissue resection in controls, staining was restricted to intracellular sources, particularly within macrophages.
D5-ethyl-βD-glucuronide was then administered to 43 individuals to establish safety and evaluate eVOC levels on breath using various breath collection and analytical approaches. Intravenous administration of the probe to subjects with lung cancer and controls revealed an excellent safety profile. The cleavage product D5-ethanol could be detected on breath in a subset of participants, and this showed a relationship with the volume of breath collected. This study demonstrates proof of mechanism for the in human cleavage of the volatile reporter molecule D5-ethanol from D5-ethyl-βD-glucuronide.
What are the next phases of this study?
The results from these proof-of-concept studies provide a promising foundation for a phase 2 dose-finding study designed to explore the diagnostic performance of this innovative breath test approach for lung cancer. We will continue our clinical study by testing a low probe concentration and optimizing the best time point for breath collection to optimize the signal-to-noise ratio of the breath test and with the aim to discriminate patients with and without lung cancer. It is important to highlight that, for the next phase, we will use an optimized method of breath collection and analysis to increase the sensitivity to detect our target VOC even further.
For this research poster, you partnered with leading institutions and organizations in the oncology space, including Cancer Research UK, the NHS, and the University of Cambridge. How important is collaboration to your work at Owlstone Medical, and how do strong relationships and partnerships accelerate challenging research areas such as cancer?
Working with others allows us to accelerate the field of breath analysis. We work closely with the key opinion leaders, ensuring we are investigating clinically relevant populations to address relevant biological questions and developing products that will impact the patient’s life positively, including in the oncology field, where we urgently need new technology for early diagnosis.
Image Credit: Giovanni Cancemi/Shutterstock.com
What is next for you and your work at Owlstone Medical in the oncology field? Are you involved in any exciting upcoming projects?
One of the exciting upcoming projects involving lung cancer diagnosis is the new research agreement with Bicycle Therapeutics plc. We will explore the development of antigen-targeted diagnostic probes that use bicyclic peptides as their targeting mechanism linked with Owlstone’s EVOC® probes. This is a proof-of-principle study for the broader opportunity by promoting the selective accumulation of the probe at the tumor for increased signal and enhanced specificity.
If successful, our work has the potential to support not only the diagnosis of the patient and speed up the work to build a more complex panel for lung cancer screening. In addition, in clinical trials, our probes can be potentially used for patient stratification. Therefore, we expect to expand to companion diagnostic tests to identify responders/non-responders for therapy selection and to measure target engagement throughout treatment.
Looking to the future, you are aiming to expand your pre-clinical system capabilities. How are you aiming to do this, and what do you hope to achieve from this expansion?
Owlstone Medical is building a unique platform to discover VOCs associated with key metabolic pathways associated with a disease but also a robust pipeline to discover new eVOC probes. We currently can evaluate liquid or air headspace VOC from in vitro or ex-vivo studies, and we are also developing different methods to collect breath samples from mice. The key point is that we use a system to trap and/or analyze VOC in our pre-clinical studies similar to the one we use for Breath Biopsy, highlighting the strong translational potential of VOC analysis from pre-clinical to clinical phases.
Strategies for VOC analysis in in vitro, ex-vivo, and in vivo animal studies can be targeted or untargeted. The untargeted approach involves measuring the whole spectrum of VOCs released in the headspace or animal breath in the presence or the absence of a disease or treatment of interest. The untargeted approach is ideal if the VOCs alterations are unknown. On the other hand, targeted approaches measure specific known compounds, generated either by cellular metabolism or by treatment with substances metabolized by metabolic pathways of interest, as our eVOC probes.
Analyzing VOCs produced by in vitro, ex-vivo, or in vivo, models can demonstrate mechanistic links between disease biology and biomarker candidates. These can subsequently be verified in clinical studies, sampling the same VOCs on breath, providing vital evidence that could bring diagnostic breath tests into clinical practice.
With increased research efforts surrounding diseases, both respiratory and cardiovascular, we are seeing scientific breakthroughs becoming more commonplace. What do you believe the future of breath biopsy to look like? Are there any particular developments you are excited to see?
Metabolic, inflammatory, and infectious diseases have the potential to be diagnosed or monitored by breath analysis. With sufficient biological understanding underpinning them, breath tests can therefore target specific metabolic pathways. With further knowledge of these pathways, we can perform targeted analysis using our platform, providing confident identification and quantification of a panel of VOCs in breath.
Using different techniques, Owlstone Medical is exploring biomarkers for different lung diseases in clinically relevant populations, including lung cancer, infection, and other inflammatory processes. I am excited to see where we can pull all these pieces together to help in the elucidation of IPN needing further management. As we are not directly assessing the location of a certain disease, as in an imaging test, we recognize that the future of a diagnostic breath biopsy test may require a panel of eVOC probes in combination with target VOC analysis to have more robust and reproducible tests.
Image Credit: SciePro/Shutterstock.com
Are you hopeful that with continued advancements surrounding breath biopsies, we will see earlier detection rates for lung cancer?
Yes! As discussed above, widespread public lung cancer screening programs mainly target the population defined as high-risk and depend on CT scanning. The success of these screening programs strongly depends on the engagement of the target population accessing the imaging sites, a widely known barrier. In addition, the screening programs for lung cancer mainly target the population currently defined as high-risk, usually defined by age, smoking, and family history. Optimization of the population to be screened still needs to be developed. Therefore, breath biopsy can help to overcome the currently limitations in lung cancer diagnosis and screening.
As well as offering your research products and services, several resources are available on your site, including publications, posters, eBooks, and blogs. Why is offering a wide variety of resources important to you?
Owlstone Medical’s vision is to be the leading source for biomarker discovery on breath, providing tools for researchers. Moreover, we are building a catalog of the validated and quantitated VOCs found on breath: the Breath Biopsy VOC Atlas, which includes insight and the scientific context of their identification. We want to provide the best discovery solutions available to our customers and maximize the chances of finding biomarkers with the potential to be translated into clinically valuable tests.
In addition, we host a Breath Biopsy Conference annually to increase the discussion and collaboration around Breath Biopsy and VOC identification.
Where can readers find more information?
Find more about the study presented at AACR 2023: https://www.owlstonemedical.com/media/uploads/files/2023-04_Evolution_poster_AACR_Update_FINAL.pdf
Find more about VOC analysis here: https://www.owlstonemedical.com/resources/
Also follow Owlstone Medical news here: https://www.owlstonemedical.com/about/news/
More about opened positions here: https://www.owlstonemedical.com/about/careers/opportunities/
About Mariana Leal
Dr Mariana Ferreira Leal is a Lead Translational Scientist at Owlstone Medical.
At the beginning of her career, Dr Leal focused on the investigation of gastric cancer diagnostic and prognostic biomarkers. She worked with gastric cancer for 15 years and led projects before moving to Royal Marsden Hospital (RMH)/The Institute of Cancer Research (ICR) in London.
She was a postdoc fellow with Dr Mitch Dowsett and then with the Dr Lesley-Ann Martin team with expertise in phenotypic and genotypic characterization of ER+ breast cancer clinical samples and in vivo and in vitro models for biomarker discovery and validation, coupled with data analysis and interpretation. She investigated the mechanisms of response and resistance to several target therapies and identified mutational and transcriptional markers that predict patients who may gain less benefit from endocrine therapies alone. She generated disease linkage data to guide indication selection and possibly targeted patient populations. She led studies with clinical trial samples as well as one longitudinal retrospective study involving patients treated at the RMH, in which she put together the cohort based on RMH databases.
This knowledge brought her to work as a Principal Scientist in a drug discovery biotech focusing on the modulation of transcription factors in breast cancer.
And now at Owlstone Medical, Dr Leal is a lead translational scientist focused on the discovery and development of non-invasive tests for lung cancer diagnosis and/or screening based on Breath Biopsy. Her role involves the discovery of new enzymatic targets and de-risk exogenous volatile organic compound (eVOC) probes to deliver biomarkers for new clinical studies focusing on non-invasive lung cancer diagnosis. She designs and leads several in vivo and in vitro studies, including using a set of biological and chemical tools to modulate the enzymatic activity of our targets. She also supports clinical trials during their completion.
Dr Leal has published extensively, with a total of 89 publications, beside book chapters and conference related documents. She is a member of various professional societies, including the eACR and ASCO.