Introduction
What is the gut virome?
Viral transplant studies
Therapeutic promise
Challenges
Conclusions
References
Further reading
Fecal virome transplantation uses viruses rather than bacteria to reshape the gut ecosystem and restore microbial balance. Early studies show it may help treat gut dysbiosis, metabolic disorders, and inflammatory conditions by transferring beneficial phage communities.
 Image Credit: Artemis / Shutterstock.com
Image Credit: Artemis / Shutterstock.com
Introduction
Fecal microbiota transplantation (FMT) involves transferring bacterial microorganisms from healthy donors to patients with a disrupted gut ecosystem, such as those with recurrent Clostridioides difficile infection. More recently, the fecal virome transplant (FVT) has emerged as an alternative approach to FMT that only transfers viruses from the stool of healthy donors to control microbial communities, with early animal studies suggesting potential benefits for treating antibiotic-associated, metabolic, and inflammatory disorders that contribute to gut dysbiosis, particularly in models where phages persistently engraft and reshape the bacterial community.4,9
What is the gut virome?
The gut virome is a diverse community of viruses that inhabit the intestinal ecosystem. The gut virome is dominated by bacteriophages that infect and regulate bacteria, along with smaller populations of eukaryotic and archaeal viruses. Although the virome accounts for only about 0.1% of the total microbial biomass within the gastrointestinal (GI) tract, it harbors more viral particles than bacterial cells, with phages comprising 90–95% of this community.2,3
From infancy, the gut is rich in phages, which diversify as diet, immunity, and environmental exposure evolve. During adulthood, the virome and bacterial microbiota exist in a dynamic equilibrium that sustains digestive and immune homeostasis.
Through their lytic and lysogenic life cycles, phages modify bacterial populations by eliminating harmful species through lysis while integrating into bacterial genomes to promote beneficial coexistence and genetic diversity.1,3 Phages exhibit immunomodulatory behavior by maintaining tolerance toward commensal bacteria while preventing infections, and evidence from mouse and human studies indicates that donor phages can engraft long-term and correlate with microbiome reshaping.4,8 Eukaryotic viruses also contribute to immune balance by regulating pathways involved in toll-like receptor (TLR) and retinoic acid-inducible gene I (RIG-I) signaling, although these effects appear more context-dependent than those of bacteriophages.3
When this equilibrium is disrupted by antibiotics, infection, or diet, phage–bacteria interactions can shift, thereby leading to dysbiosis, inflammation, and altered immunity. Emerging research suggests that the gut virome plays a crucial role in both intestinal and systemic immune regulation, with potential implications for treating inflammatory bowel disease (IBD) and obesity; however, its influence on outcomes such as cancer immunotherapy remains preliminary and requires further validation.3
Viral transplant studies
In mice, transferring viral particles from healthy donors reconstructed the gut microbiome after antibiotic treatment, thereby reshaping microbial diversity and preventing dysbiosis.4 FVT enriched with Akkermansia muciniphila has also been shown to promote the growth of this beneficial bacterium in recipients and improve fertility outcomes.5
In preterm piglets, FVT from healthy donors prevented necrotizing enterocolitis (NEC), a life-threatening intestinal disease, by increasing viral diversity and reducing pathogenic Proteobacteria. In contrast, conventional FMT did not confer the same protection, thus highlighting the unique therapeutic potential of viral fractions.6
Pilot studies in humans provide early evidence of efficacy. In one small trial, sterile fecal filtrates containing only viruses successfully reestablished normal bowel function for at least six months in patients with recurrent Clostridioides difficile infection (CDI), with virome engraftment correlating with symptom resolution.7,8
Several studies also demonstrate that phage engraftment is highly host-specific, with transferred phages proliferating only when compatible bacterial hosts are present, emphasizing the importance of donor–recipient matching at the microbial level.4,11
 Image Credit: paulista / Shutterstock.com
Image Credit: paulista / Shutterstock.com
Therapeutic promise
In both obese and lean mice, FVT improved insulin sensitivity, reduced body weight, and normalized glucose metabolism, all of which are results of enhanced expression of metabolic genes that control the activity of leptin receptors (LEPR), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A), and insulin-like growth factor-binding protein 2 (IGFBP2). This treatment also normalized bacterial diversity, suggesting that phages can reprogram host metabolism by reconstituting bacterial communities and regulating key pathways involved in glucagon-like peptide-1 (GLP-1) and leptin signaling.9,10
Modified FVT approaches, such as chemostat-propagated viromes that exclude eukaryotic viruses, have similarly improved blood glucose levels.10 In one recent study, FVT from donors consuming different diets altered recipient microbiota and reliably shifted body-weight phenotypes in mice across independent experiments, thus exemplifying the reproducibility of virome-driven phenotypic editing and diet–virome interactions.11
In IBD, virome imbalance, particularly the expansion of Caudovirales phages and a concurrent reduction in Microviridae abundance, contributes to dysregulated immune activation through TLR-9 and interferon-gamma (IFN-γ) pathways. By reinstating phage diversity and reducing harmful viral overgrowth, FVT could rebalance bacterial populations and reduce the intensity of immune responses, although patterns of virome alteration vary across IBD cohorts and sampling sites.13
In septic mice, transfer of fecal viral communities reduced inflammation and selectively lowered Proteobacteria/Gammaproteobacteria and other taxa. Oral administration of isolated phages similarly led to anti-inflammatory effects, supporting phage-mediated remodeling of dysbiotic communities.14
Recent studies further show that these effects may depend on the stability and persistence of donor phages in the gut, with some phages demonstrating long-term colonization while others remain transient.8,11
Challenges
The clinical translation of FVT is limited by safety concerns, particularly the potential for pathogenic eukaryotic virus transmission. Although donor material is sterile-filtered, this process may not eliminate pathogenic viruses like papillomaviruses, herpesviruses, hepatitis viruses, enteroviruses, or noroviruses, especially non-enveloped RNA viruses that tolerate chemical and filtration steps.15
To mitigate these risks, several modified FVT techniques have been developed, including solvent–detergent treatment (FVT-SDT) to inactivate enveloped viruses, pyronin-Y treatment (FVT-PyT) to suppress RNA virus activity, and chemostat-propagated viromes (FVT-ChP) that dilute or remove eukaryotic viral contaminants while preserving bacteriophages.2
The lack of standardized methods for virome isolation, sequencing, and delivery is an additional challenge associated with FVT. Virome sequencing is technically demanding, with traditional next-generation sequencing offering limited resolution and high background noise. Emerging long-read and ultra-deep metagenomic methods provide improved viral genome reconstruction and may standardize future FVT workflows.2,15
Donor material variability, unclear classification as a biological product or therapeutic agent, and limited patient acceptance further complicate the adoption of FVT. Recent discussions emphasize the need for explicit regulatory frameworks distinguishing between whole-microbiota and phage-enriched products, as well as harmonized screening standards and ethical oversight to address long-term risks.15
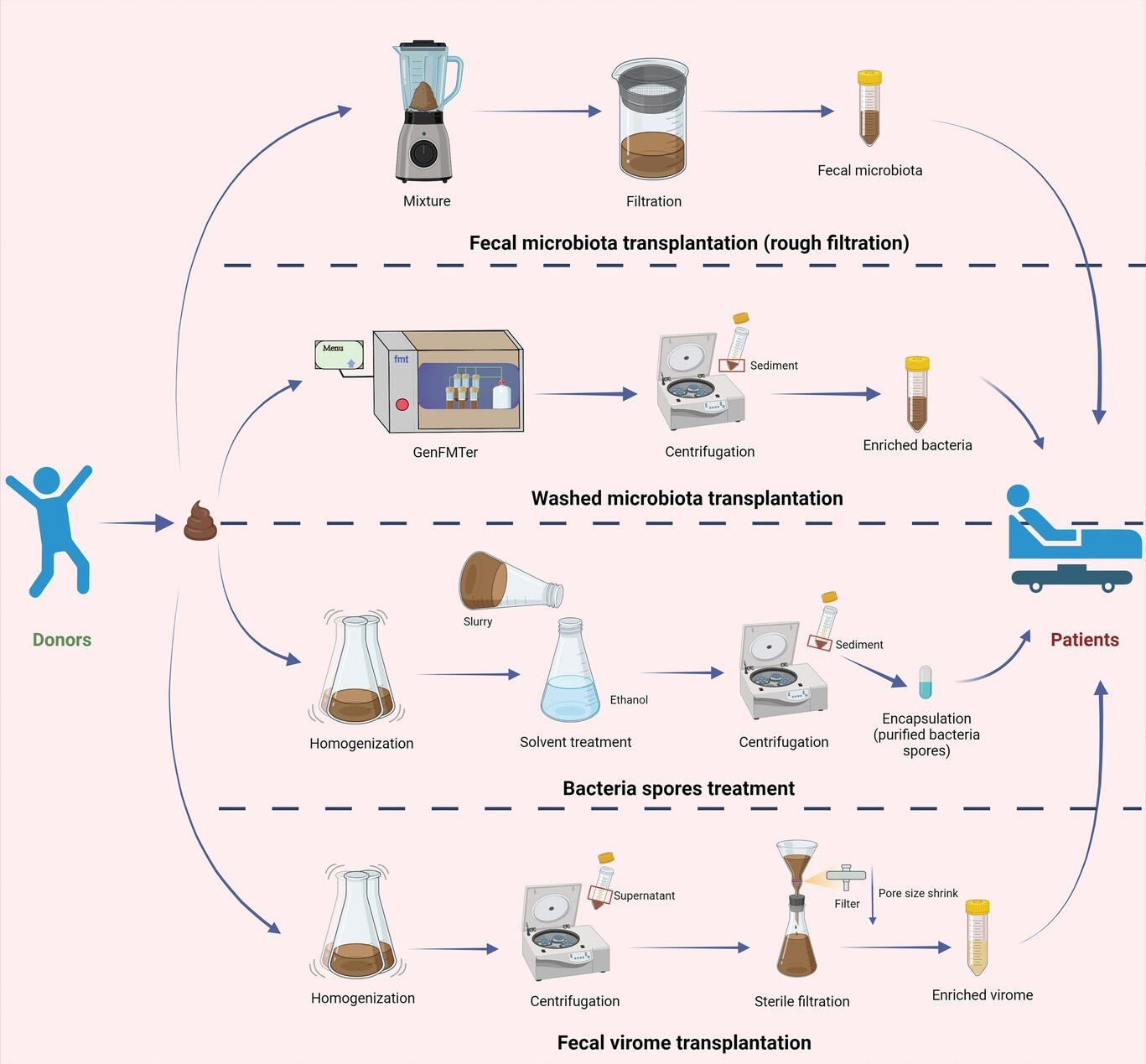
The conceptual graphs of preparation methodology for fecal transplants. The preparation methods for fecal transplants: fecal microbiota transplantation (rough filtration), washed microbiota transplantation, bacteria spores treatment, and fecal virome transplantation.15
Conclusions
By selectively targeting harmful bacteria while preserving beneficial microorganisms, FVT offers a safer and more refined alternative to traditional FMT, particularly when enriched for bacteriophages and processed to reduce eukaryotic viral content, and when donor–recipient phage–host compatibility is considered.1,2 As research advances, the targeted manipulation of viral ecosystems could redefine microbiome-based medicine and transform the management of gut and metabolic disorders.
References
- Zeng, C., Wan, S., Guo, M., et al. (2024). Fecal virome transplantation: A promising strategy for the treatment of metabolic diseases. Biomedicine & Pharmacotherapy 177; 117065. DOI:10.1016/j.biopha.2024.117065, https://www.sciencedirect.com/science/article/pii/S0753332224009491.
- Rasmussen, T. S., Mao, X., Forster, S., et al. (2024). Overcoming donor variability and risks associated with fecal microbiota transplants through bacteriophage-mediated treatments. Microbiome 12; 119. DOI:10.1186/s40168-024-01820-1, https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-024-01820-1.
- Feng, Z., Burgermeister, E., Philips, A., et al. (2025). The gut virome in association with the bacteriome in gastrointestinal diseases and beyond: Roles, mechanisms, and clinical applications. Precision Clinical Medicine 8(2), pbaf010. DOI:10.1093/pcmedi/pbaf010, https://academic.oup.com/pcm/article/8/2/pbaf010/8152264.
- Draper, L.A., Ryan, F. J., Dalmasso, M. et al. (2020). Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biology 18; 173. DOI:10.1186/s12915-020-00906-0, https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-020-00906-0.
- Rasmussen, T. S., Mentzel, C. M. J., Danielsen, M. R., et al. (2023). Fecal virome transfer improves proliferation of commensal gut Akkermansia muciniphila and unexpectedly enhances the fertility rate in laboratory mice. Gut Microbes 15(1). DOI:10.1080/19490976.2023.2208504, https://www.tandfonline.com/doi/10.1080/19490976.2023.2208504.
- Brunse, A., Deng, L., Pan, X., et al. (2022). Fecal filtrate transplantation protects against necrotizing enterocolitis. The ISME Journal 16(3); 686–694. DOI:10.1038/s41396-021-01107-5, https://www.nature.com/articles/s41396-021-01107-5.
- Ott, S. J., Waetzig, G. H., Rehman, A., et al. (2017). Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients with Clostridium difficile Infection. Gastroenterology 152(4); 799–811. DOI:10.1053/j.gastro.2016.11.010, https://www.gastrojournal.org/article/S0016-5085(16)35354-9/fulltext.
- Wortelboer, K., de Jonge, P. A., Scheithauer, T. P. M., et al. (2023). Phage-microbe dynamics after sterile faecal filtrate transplantation in individuals with metabolic syndrome: a double-blind, randomised, placebo-controlled clinical trial assessing efficacy and safety. Nature Communications 14; 5600. DOI:10.1038/s41467-023-41329-z, https://www.nature.com/articles/s41467-023-41329-z.
- Rasmussen, T. S., Mentzel, C. M. J., Kot, W., et al. (2020). Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 69; 2122–2130. DOI:10.1136/gutjnl-2019-320005, https://gut.bmj.com/content/69/12/2122.long.
- Mao, X., Larsen, S. B., Zachariassem, L. S. F., et al. (2024). Transfer of modified gut viromes improves symptoms associated with metabolic syndrome in obese male mice. Nature Communications 15; 4704. DOI:10.1038/s41467-024-49152-w, https://www.nature.com/articles/s41467-024-49152-w.
- Borin, J. M., Liu, R., Wang, Y., et al. (2023). Fecal virome transplantation is sufficient to alter fecal microbiota and drive lean and obese body phenotypes in mice. Gut Microbes 15(1). DOI:10.1080/19490976.2023.2236750, https://www.tandfonline.com/doi/10.1080/19490976.2023.2236750.
- Lin, D. M., Koskella, B., Ritz, N. L., et al. (2019). Transplanting Fecal Virus-Like Particles Reduces High-Fat Diet-Induced Small Intestinal Bacterial Overgrowth in Mice. Frontiers in Cellular and Infection Microbiology 9. DOI:10.3389/fcimb.2019.00348, https://www.frontiersin.org/articles/10.3389/fcimb.2019.00348.
- Liang, G., Cobián-Güemes, A. G., Albenberg, L., & Bushman, F. (2021). The gut virome in inflammatory bowel diseases. Current Opinion in Virology 51; 190–198. DOI:10.1016/j.coviro.2021.10.005, https://www.sciencedirect.com/science/article/pii/S1879625721001279.
- Chancharoenthana, W., Sutnu, N., Visitchanakun, P., et al. (2022). Critical roles of sepsis-reshaped fecal virota in attenuating sepsis severity. Frontiers in Immunology 13, 940935. DOI:10.3389/fimmu.2022.940935, https://www.frontiersin.org/articles/10.3389/fimmu.2022.940935.
- Yu, Y., Wang, W., & Zhang, F. (2023). The Next Generation Fecal Microbiota Transplantation: To Transplant Bacteria or Virome. Advanced Science 10(35). DOI:10.1002/advs.202301097, https://onlinelibrary.wiley.com/doi/10.1002/advs.202301097.
Further Reading
Last Updated: Nov 23, 2025