Introduction
Etiology and transmission
Pathogenesis and clinical features
Epidemiology and recent outbreaks
Diagnosis and treatment
Public health concerns and risks
Prevention and control measures
Conclusions and future outlook
References
Further reading
Explore how plague defies its ancient reputation and why coordinated science and public health responses are still needed to stop its spread.
 Image Credit: Kateryna Kon / Shutterstock.com
Image Credit: Kateryna Kon / Shutterstock.com
Introduction
Plague, which is caused by infection with Yersinia pestis, has shaped human history through three major pandemics. The bacterium is a descendant of Yersinia pseudotuberculosis and has diversified into several biovars (Antiqua, Orientalis, Medievalis, and Microtus), each associated with specific outbreaks and geographic regions.
Despite its reputation as a disease of the past, cases of the plague still arise from entrenched animal reservoirs, in addition to reports of multidrug resistance and the bioterrorism potential of this pathogen, thus emphasizing the importance of sustained surveillance and preparedness. Natural plague foci remain distributed in rodent populations worldwide, making eradication impossible and periodic spillover into humans inevitable1,2.
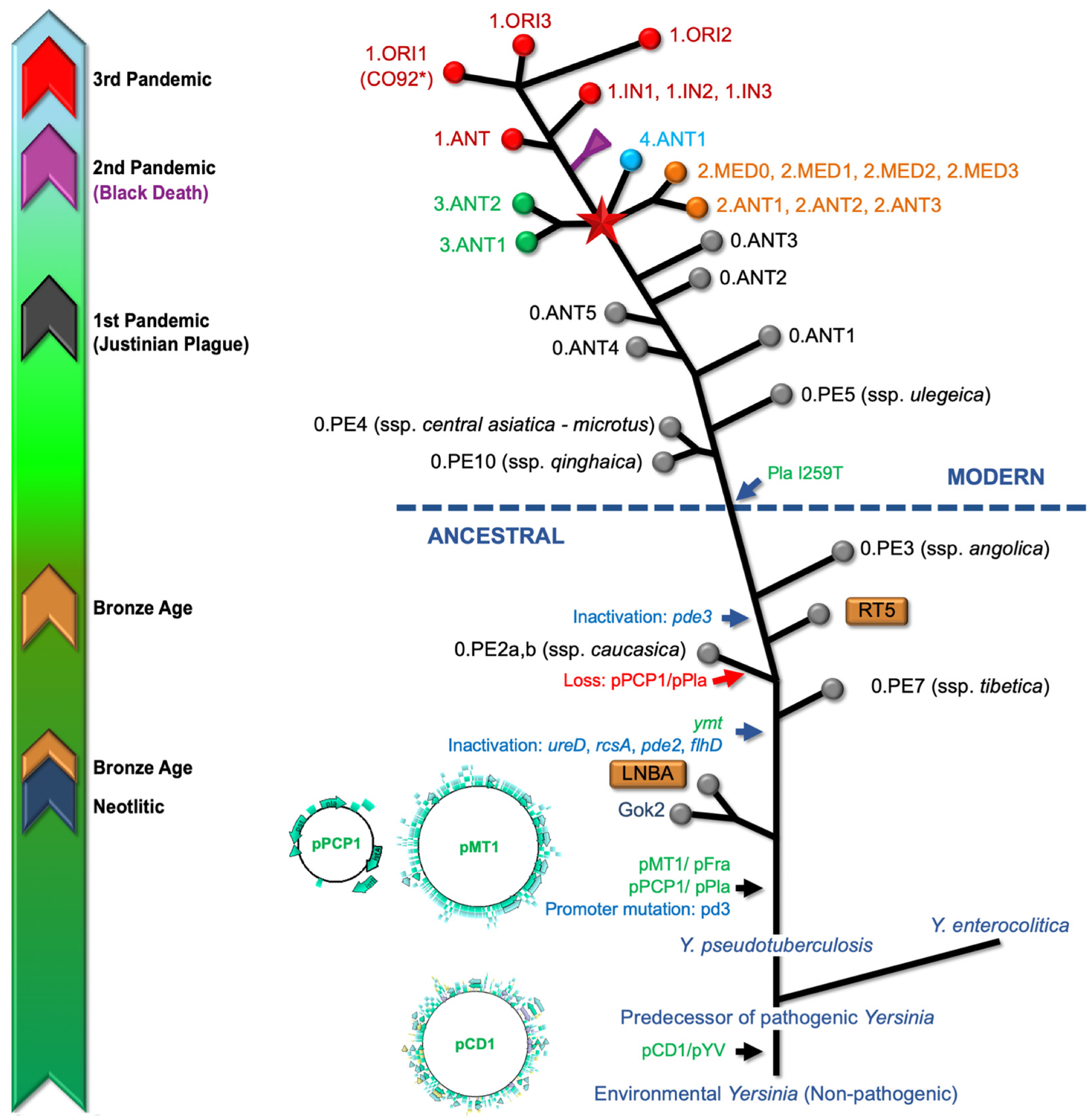 Graphic representation of the Yersinia pestis genealogy based on the consensus information recompiled from several sources. The timely historical guide (left) refers to registered periods for aDNA specimens and recorded plague pandemics. The enteropathogenic Yersinia species, including Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis harbors the pCD1/pYV, an important 70-kb virulence plasmid that plays a key role in facilitating infection, circumventing host defense mechanisms. The acquisition of this plasmid implied a change from an hypothetical non-pathogenic environmental Yersinia into a predecessor of the pathogenic members of this species. The acquisition of two virulence-associated plasmids (pMT1, pPCP1) and chromosome rearrangement processes seems that turned Y. pseudotuberculosis into Y. pestis. Additional significant genetic events have been observed throughout the evolutionary trajectory of Y. pestis, including the acquisition of the Yersinia murine toxin (ymt) gene, within the pMT1 plasmid and the inactivation of genes associated with virulence. The extinct Neolithic (Gok2) and Bronze Age lineages (LNBA and RT5) are included). Genomic comparative analyses indicate that the I259 Pla isoform is an ancient variant, so the acquisition of the I259T mutation in the plasminogen activator, occurring after the 0.PE3 (ssp. angolica) strain, is a distinctive feature characterizing the so-called modern strains. The node, indicated by a red star, represents the so-called “Big Bang” event in the evolutionary history of Y. pestis; which led to the emergence of Branches 1 to 4 and significantly contributed to the existing strain diversity, including lineages associated with the Modern plague pandemic. Abbreviations: ANT (Antiqua), MED (Medievalis), ORI (Orientalis), IN (Intermediate), PE (Pestoides). Several branches have been collapsed for clarity, including the Black Death and Intermediate strains clusters of Branch 1 and Branch 2 (Antiqua and Medievalis). Colors codes used for circles at the branch ends are (Microorganisms 12 00146 i001) Branch 0, (Microorganisms 12 00146 i002) Branch 1, (Microorganisms 12 00146 i003) Branch 2, (Microorganisms 12 00146 i004) Branch 3, and (Microorganisms 12 00146 i005) Branch 4. Branch length does not represent the evolutionary time. * The genome of Y. pestis, strain CO92 (including plasmids), is usually used as a reference for read mapping from aDNA.
Graphic representation of the Yersinia pestis genealogy based on the consensus information recompiled from several sources. The timely historical guide (left) refers to registered periods for aDNA specimens and recorded plague pandemics. The enteropathogenic Yersinia species, including Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis harbors the pCD1/pYV, an important 70-kb virulence plasmid that plays a key role in facilitating infection, circumventing host defense mechanisms. The acquisition of this plasmid implied a change from an hypothetical non-pathogenic environmental Yersinia into a predecessor of the pathogenic members of this species. The acquisition of two virulence-associated plasmids (pMT1, pPCP1) and chromosome rearrangement processes seems that turned Y. pseudotuberculosis into Y. pestis. Additional significant genetic events have been observed throughout the evolutionary trajectory of Y. pestis, including the acquisition of the Yersinia murine toxin (ymt) gene, within the pMT1 plasmid and the inactivation of genes associated with virulence. The extinct Neolithic (Gok2) and Bronze Age lineages (LNBA and RT5) are included). Genomic comparative analyses indicate that the I259 Pla isoform is an ancient variant, so the acquisition of the I259T mutation in the plasminogen activator, occurring after the 0.PE3 (ssp. angolica) strain, is a distinctive feature characterizing the so-called modern strains. The node, indicated by a red star, represents the so-called “Big Bang” event in the evolutionary history of Y. pestis; which led to the emergence of Branches 1 to 4 and significantly contributed to the existing strain diversity, including lineages associated with the Modern plague pandemic. Abbreviations: ANT (Antiqua), MED (Medievalis), ORI (Orientalis), IN (Intermediate), PE (Pestoides). Several branches have been collapsed for clarity, including the Black Death and Intermediate strains clusters of Branch 1 and Branch 2 (Antiqua and Medievalis). Colors codes used for circles at the branch ends are (Microorganisms 12 00146 i001) Branch 0, (Microorganisms 12 00146 i002) Branch 1, (Microorganisms 12 00146 i003) Branch 2, (Microorganisms 12 00146 i004) Branch 3, and (Microorganisms 12 00146 i005) Branch 4. Branch length does not represent the evolutionary time. * The genome of Y. pestis, strain CO92 (including plasmids), is usually used as a reference for read mapping from aDNA.
Etiology and transmission
Yersinia pestis, the etiologic agent of plague, is a Gram‑negative, facultative intracellular coccobacillus that multiplies within macrophages. This pathogen neutralizes innate immune responses through a type III secretion system that facilitates rapid dissemination. Specific virulence factors include the Pla protease and Yersinia outer proteins (Yops), which help the bacterium evade host defenses2.
Primary pneumonic plague occurs after inhalation of infectious droplets from a coughing patient. Comparatively, secondary pneumonic plague develops when an untreated bubonic or systemic infection reaches the lungs. The incubation period for primary pneumonic plague is short, typically two to four days after exposure, but can range from one to six days depending on infectious dose and route5.
Droplet spread is most efficient late in the illness; however, this form of transmission requires close face‑to‑face contact with a distance of less than one meter for prolonged durations. Rare airborne transmission over greater distances has been reported, but is exceptional6.
In addition to respiratory droplets, plague can be transmitted by flea bites (the most common route for bubonic plague), direct handling of infected animal tissues, contact with contaminated bodily fluids, and rarely by ingestion of undercooked meat from infected animals (gastrointestinal and pharyngeal forms)2,5.
Across documented outbreaks, each pneumonic case infects an average of 1.3 secondary cases, which exemplifies its relatively narrow contagion window. Field investigations confirm this low efficiency, as two index patients in Uganda transmitted the infection to one caregiver each, with none of the other 23 untreated close contacts developing infection. However, in crowded or closed settings, the basic reproductive number (R0) may exceed 1, allowing for sustained person-to-person transmission in some outbreaks2,6.
Y. pestis does not persist long in the environment; it is rapidly killed by ultraviolet light, temperatures above 40°C, or desiccation2.
Pathogenesis and clinical features
Within the first 24 hours after infection, bacterial replication is modest, and patients may be relatively non-infectious. Over the next 24-72 hours, bacterial burdens rapidly rise, with these pathogens detectable in both sputum and blood.
Clinical deterioration is abrupt and may include high fever, cough with bloody sputum, dyspnea, pleuritic chest pain, and tachycardia. Respiratory failure typically causes death, rather than primary septic shock.
Yersinia pestis invades the alveolar spaces and rapidly enters the vascular system to induce fulminant necrotizing pneumonia with severe alveolar edema and vascular hemorrhage. Histology shows destruction of alveolar architecture, intense inflammation, macrophage recruitment with apoptosis, and diffuse interstitial pneumonia, thereby reflecting the impact of the type III secretion system on host cells.3
Without immediate antimicrobial therapy, which is ideally initiated within about 12 hours of fever onset, mortality is almost inevitable. By 36-48 hours after infection, high bacterial titers are detectable and may be accompanied by liquefactive necrosis, polymorphonuclear infiltration, and diffuse interstitial pneumonia. Thereafter, bacteremia, hepatic and splenic involvement, as well as a delayed inflammatory response, will immediately precede death.3
The main clinical forms of plague are bubonic (most common, characterized by painful lymphadenopathy or "buboes"), pneumonic (primary or secondary, with rapidly progressing pneumonia), and septicemic (primary or secondary bloodstream infection). Rare forms include pharyngeal, gastrointestinal, cutaneous, meningitic, and endophthalmitic plague2,5.
Untreated bubonic plague has a case-fatality rate of 40–70%, untreated pneumonic plague is nearly always fatal, and septicemic plague carries high mortality. With prompt and appropriate treatment, overall case-fatality rates fall to 5–15% for bubonic and much lower for other forms2.
Plague 101 | National Geographic
Epidemiology and recent outbreaks
Plague remains globally distributed; however, Madagascar, the Democratic Republic of the Congo, and certain regions throughout Asia are most commonly affected by these outbreaks. Over 90% of human plague cases worldwide occur in Africa, with Madagascar, DRC, and Peru among the most endemic countries2,7.
Each year, hundreds to a few thousand human cases of the plague are reported throughout the world, with Madagascar alone reporting about 1,500 confirmed cases in some years, but case numbers fluctuate and significant underreporting is suspected4,5,7.
Over the past two decades, several pneumonic plague outbreaks were documented near Antananarivo in 1997, Uganda in 2004, as well as 87 and 117 cases reported in the Democratic Republic of the Congo in 2005 and 2006, respectively. A major urban outbreak in Madagascar in 2017 led to 2,417 confirmed cases, mostly pneumonic, with rapid spread in crowded settings. These patterns emphasize the crucial need to continue monitoring endemic transmission of the plague despite modern antibiotics and vector control programs.4,7
Surveillance gaps and underreporting remain major problems, especially in rural or politically unstable regions where access to care and laboratory confirmation are limited. In 2011, a rapid outbreak in remote northern Madagascar was suspected to have infected 17 individuals, along with two presumptive and three confirmed cases. All 15 untreated patients died within 27 days.4
Field teams arrived two months after the first case was reported, which exemplifies how geography and poor infrastructure can delay recognition and response. The detection of streptomycin‑resistant Yersinia pestis in Madagascar further increases epidemiologic risk while reinforcing the need for sustained and well‑resourced surveillance systems that can quickly detect and contain outbreaks.4
Diagnosis and treatment
Rapid diagnosis and treatment are critical in pneumonic plague. Clinicians should collect sputum, blood, or bubo aspirates for culture, staining, polymerase chain reaction (PCR) assays, and serology for the fraction 1 (F1) antigen or a rise in anti‑F1 antibodies. When laboratory capabilities are limited, rapid immunochromatographic tests provide point‑of‑care confirmation. Loop-mediated isothermal amplification (LAMP) and lateral-flow immunoassays are additional rapid diagnostic tools suitable for outbreak and field settings5.
Chest imaging supports recognition of pneumonic involvement. The World Health Organization (WHO) framework classifies cases as suspected, presumptive, or confirmed based on clinical-epidemiologic features plus laboratory evidence.5
Delaying treatment beyond 24 hours is often fatal; therefore, treatment should be initiated as soon as possible. Streptomycin or gentamicin are first‑line antibiotics, including for pregnant and immunocompromised patients, with pediatric dose adjustments also available. Other antibiotics with proven efficacy include doxycycline, tetracycline, ciprofloxacin, and levofloxacin. Chloramphenicol is used for severe illness or meningitis2,5.
Doxycycline and ciprofloxacin are effective alternatives and preferred oral options during large outbreaks or bioterrorism events. Chloramphenicol may also be incorporated into the treatment plan for patients experiencing severe illness.
Although most Yersinia pestis isolates remain susceptible, a multidrug‑resistant strain has been reported. Supportive care, including oxygenation or mechanical ventilation for respiratory failure, fluid resuscitation, vasopressors, and shock management, is essential to improve survival. Historical or experimental adjunctive therapies such as phage therapy, serum therapy, and bacteriocins have been explored but are not standard of care2,5.
Public health concerns and risks
In 2017, about 78% of patients infected during a plague outbreak in Madagascar were pneumonic, many of whom resided in a small urban area, which confirms the rapid transmission potential in crowded settings.
Through the use of the WHO classic worst‑case model, researchers estimate that releasing 50 kg of Yersinia pestis over a city of five million could infect about 150,000 people and kill 36,000 people. Accordingly, the United States Centers for Disease Control and Prevention (CDC) classifies Yersinia pestis as a Category A bioterrorism agent that demands special preparedness because it can spread person to person, as well as cause high mortality and public panic. Modern preparedness strategies also include stockpiling antibiotics and laboratory diagnostics6,7.
Multidrug‑resistant Yersinia pestis strains carrying plasmids pIP1202 and pIP1203 have already been described in Madagascar, with resistance determinants capable of being transferred to Escherichia coli. Reportedly, the former Soviet Union engineered multidrug‑ and fluoroquinolone‑resistant strains, which demonstrates the feasibility of deliberate enhancement.
Other factors that increase the risk of antibiotic resistance include spontaneous genetic mutations, climate‑change-driven disruption of natural foci, and deliberate aerosol release, particularly where surveillance, antibiotics, and protective equipment are limited. Despite these threats, the majority of Y. pestis isolates globally remain susceptible to standard antibiotics. Animal and environmental surveillance, particularly monitoring rodents and fleas, is a cornerstone of public health control1,5,6,7.
 Image Credit: Pordee_Aomboon / Shutterstock.com
Image Credit: Pordee_Aomboon / Shutterstock.com
Prevention and control measures
Rapid case identification, isolation, contact tracing, targeted chemoprophylaxis, and rigorous vector–reservoir control are essential for preventing transmission of the plague. Suspected or confirmed pneumonic cases, including bubonic cases with secondary pulmonary involvement, should be isolated under droplet precautions, preferably in negative‑pressure rooms, with staff wearing gowns, gloves, surgical masks, and eye protection.
Post‑exposure antibiotics like tetracycline or trimethoprim-sulfamethoxazole should be prescribed to people bitten by fleas, exposed to infected animal tissues/fluids, household contacts of bubonic patients, or close contacts of pneumonic patients. In mass‑casualty settings, oral doxycycline, tetracycline, or ciprofloxacin is recommended.2,5
The WHO has endorsed plague prophylaxis with tetracycline, streptomycin, and chloramphenicol; however, the real-world application of this treatment protocol varies. To date, no vaccine is currently recommended by the WHO or the United States CDC. Live attenuated vaccines are used in some countries (e.g., Russia); a killed whole-cell vaccine exists but is not widely available. Newer recombinant and DNA vaccines are under development, but no proven effective or widely licensed vaccine for plague prevention is available as of 20242,5.
In endemic zones, rodent and flea control is essential, in addition to removing food/garbage, treating pets for fleas, and using licensed insecticides, rodenticides, or traps, while sentinel carnivore surveillance offers early warning. Simple source control, such as wearing face masks, further reduces pneumonic transmission.2,5
Conclusions and future outlook
Plague remains a persistent zoonosis threat that can cause lethal pneumonic outbreaks despite modern antibiotics and intensive care. Sustained, interoperable surveillance linking human, veterinary, and entomologic data, rapid point-of-care diagnostics, and preplanned surge capacity for isolation, contact tracing, and prophylaxis are essential to reduce the risk of large outbreaks.
Continued research is needed to deliver safe and effective vaccines against primary pneumonic disease. Investing in the development of field-deployable assays and the discovery of new antimicrobials or combinations that can evade multidrug resistance is also crucial. Maintaining public health infrastructure, especially in endemic regions, is vital for early detection and effective response. Expanding molecular and genetic screening in animal populations and addressing gaps in ecological and social determinants of outbreaks remain priorities for future research1.
References
- Bennasar-Figueras, A. (2024). The Natural and Clinical History of Plague: From the Ancient Pandemics to Modern Insights. Microorganisms, 12(1). DOI:10.3390/microorganisms12010146, https://www.mdpi.com/2076-2607/12/1/146
- Barbieri, R., Signoli, M., Chevé, D., Costedoat, C., Tzortzis, S., Aboudharam, G., Raoult, D. and Drancourt, M. (2020). Yersinia pestis: the natural history of plague. Clinical microbiology reviews, 34(1). DOI:10.1128/cmr.00044-19, https://journals.asm.org/doi/10.1128/cmr.00044-19
- Anderson, D.M., Ciletti, N.A., Lee-Lewis, H., Elli, D., Segal, J., DeBord, K.L., Overheim, K.A., Tretiakova, M., Brubaker, R.R. and Schneewind, O. (2009). Pneumonic plague pathogenesis and immunity in Brown Norway rats. The American journal of pathology, 174(3), 910-921. DOI:10.2353/ajpath.2009.071168, https://www.sciencedirect.com/science/article/pii/S0002944010609509
- Richard, V., Riehm, J.M., Herindrainy, P., Soanandrasana, R., Ratsitoharina, M., Rakotomanana, F., Andrianalimanana, S., Scholz, H.C. and Rajerison, M. (2015). Pneumonic Plague Outbreak, Northern Madagascar, 2011. Emerging Infectious Diseases, 21(1), 8-15. DOI:10.3201/eid2101.131828, https://wwwnc.cdc.gov/eid/article/21/1/13-1828_article
- Yang R. (2018). Plague: Recognition, Treatment, and Prevention. J Clin Microbiol. 56(1). DOI:10.1128/jcm.01519-17, https://journals.asm.org/doi/10.1128/jcm.01519-17
- Evans, C. (2022). Pneumonic Plague: Incidence, Transmissibility and Future Risks. Hygiene, 2(1), 14-27. DOI:10.3390/hygiene2010002, https://journals.asm.org/doi/10.1128/jcm.01519-17
- Ditchburn, J. L., & Hodgkins, R. (2019). Yersinia pestis, a problem of the past and a re-emerging threat. Biosafety and Health, 1(2), 65-70. DOI:10.1016/j.bsheal.2019.09.001, https://www.sciencedirect.com/science/article/pii/S2590053619300230
Further Reading
Last Updated: Jul 31, 2025