The citric acid cycle is also known as the Krebs cycle or the tricarboxylic acid cycle. It is a series of reactions in a closed loop that are fundamental for cellular respiration. The citric acid cycle produces the high-energy molecule ATP (adenosine triphosphate) and byproducts that also form ATP in a further process called oxidative phosphorylation.
Credit: Borbely Edit/Shutterstock.com
Regulation of the citric acid cycle is important as reactions that are unchecked will lead to large amounts of wasted metabolic energy. The ability to regulate the cycle keeps the cell in a stable state, and this function is maintained by three mechanisms:
- The availability of substrates.
- Inhibition of the products formed.
- Inhibition of enzymes through allosteric feedback.
Regulation of acetyl CoA
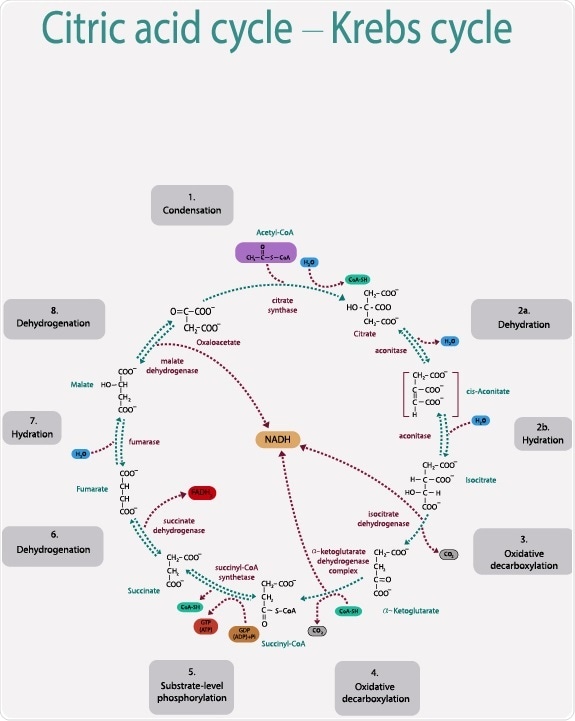
The citric acid cycle begins with the reaction that combines the two-carbon acetyl CoA with a four-carbon oxaloacetic acid to produce the six-carbon molecule citrate. Acetyl-CoA is regulated by the controlled amounts of pyruvate that is converted into acetyl-CoA in the pyruvate dehydrogenase complex reaction.
Metabolite flow is allosterically inhibited, where an enzyme is regulated by binding an effector molecule to a non-active site. The pyruvate dehydrogenase complex reaction is allosterically inhibited when there are high ratios of ATP to ADP, NADH to NAD+ and acetyl-CoA to CoA. Allosteric activation occurs when the ratio volumes decrease.
Regulation of enzymes in the citric acid cycle
Three reactions of the cycle are catalyzed respectively by the enzymes:
- Citrate synthase.
- Isocitrate dehydrogenase.
- α-ketoglutarate dehydrogenase
Citrate synthase is responsible for the rate of reaction in the first step of the cycle when the acetyl-CoA is combined with oxaloacetic acid to form citrate. It is inhibited by high concentrations of ATP, acetyl-CoA, and NADH which indicates an already high level of energy supply. The molecule produced in the reaction, citrate, can also act as an inhibitor of the reaction.
Because citrate synthase is inhibited by the final product of the citric acid cycle as ATP, ADP (adenosine diphosphate) works as an allosteric activator of the enzyme as ATP is formed from ADP. Therefore, the rate of the cycle is reduced when the cell has a high level of ATP.
The enzyme isocitrate dehydrogenase is an important catalyst in the third step of the reaction. It regulates the speed at which the citrate isomer isocitrate loses a carbon to form the five-carbon molecule α-ketoglutarate. The coenzyme NADH is a product of the reaction and, at high levels, acts as an inhibitor by directly displacing the NAD+ molecules it is formed from.
The enzyme α-ketoglutarate dehydrogenase is another important catalyst in the fourth step of the cycle where α-ketoglutarate also loses a carbon and combines with Coenzyme A to form succinyl CoA. The two products of the reaction, succinyl CoA and NADH, both work as inhibitors at large concentrations.
Calcium as a regulator of the citric acid cycle
Calcium is also an important regulator of the citric acid cycle; an increase in concentrations of both ADP and calcium ions (Ca2+) are a consequence of changes in cellular activity. Therefore, the signal that stimulates muscle contraction is also activating the production of the ATP which sustains it, through the citric acid cycle. Calcium ions regulate the citric acid cycle by activating pyruvate dehydrogenase, the first component of the pyruvate dehydrogenase complex reaction that forms acetyl-CoA. Calcium ions also activate the enzymes, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase which catalyze the third and fourth steps of the cycle respectively. The activation of these enzymes, via calcium ions, increases the rate of separate reactions within the cycle and therefore increases product production for the whole cycle.
Sources:
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 17.2, Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled. https://www.ncbi.nlm.nih.gov/
- Wan, B. et al. 1989. Regulation of citric acid cycle by calcium, Journal of Biological Chemistry, 264, pp. 13430-13439. https://www.ncbi.nlm.nih.gov/pubmed/2503501
- Traaseth, N. et al. 2004. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1658, pp.64-71. http://www.sciencedirect.com/science/article/pii/S0005272804001343
Further Reading
Last Updated: Jul 19, 2023