Defining Keto- and Enol Tautomerism
Ketones are in equilibrium with a form known as an enol. The name enol derives from the fact that enols are a combination of a carbonyl (C=O) containing group, such as an aldehyde or ketone and an alcohol hydroxyl (OH) group. The interconversion between these two forms arises from a process called tautomerism.
Ketones and enols are isomers. Isomers are molecules that are made of the same atoms, but differ in their connectivity.
These two isomers are related by a change in the position of the hydrogen and the double bond in the molecule. Thus, tautomerism describes the equilibrium between keto and enol forms interconverted through a change in the position of bonding electrons and hydrogen to produce two isomers.
This process is typical of ketones, aldehydes and esters, and in general, the interconversion is slow. The isomer containing the carbonyl compound is favoured over the enol form, with the keto form dominating at >99%. Manipulation of these proportions can be achieved through catalysis, specifically by acids or bases.
The Formation of Cyclic Sugars
Sugars are polyhydroxylated (containing many OH groups) chains of carbon atoms that additionally feature an aldehyde or ketone functional group.
In general, all sugars feature a 1:2:1 ratio of carbon: hydrogen: oxygen. Subsequently, carbohydrates possess the ability to undergo internal, or intramolecular, reactions between the reactive hydroxyl group and the carbonyl carbon.
The hydroxyl is nucleophilic; it possesses a lone electron pair that it can donate to the carbonyl carbon and thus form a bond. The carbonyl carbon is amenable to such bond formation as its electrons experience a pulling effect by the doubly-bonded oxygen. Oxygen can attract the bonding pair of electrons more strongly than carbon; it is said to be more electronegative.
When a single nucleophilic hydroxyl group in a sugar attacks an aldehyde or ketone, a product called a hemiacetal or hemiketal, respectively, is produced. The prefix hemi- denotes the reversibility of the reaction. They are also referred to as acetals or pyranoses.
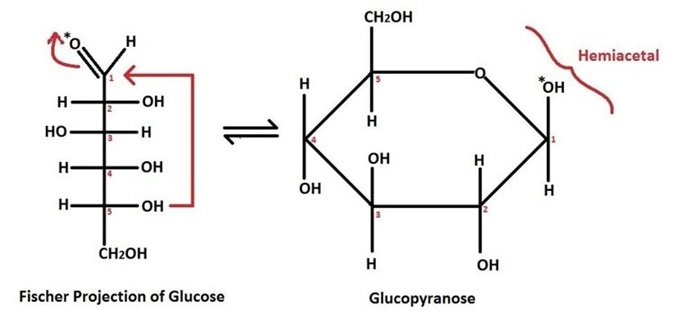
Figure 1 Example of a cyclisation reaction with D-glucose. When an aldose cyclizes, the hydroxyl group on C5 undergoes an intramolecular reaction with the C1 carbonyl group of the aldehyde. The product formed is a hemiacetal. Note the two-dimensional representation of the sugar D-glucose on the left-hand side is called a Fischer Projection, the resultant 3D representation of the hemiacetal is called a Haworth Projection.
These are cyclic, due to the ring-closing effect produced by chemical bond formation. Note that an attack on an aldehyde to produce a hemiacetal results in a 6-membered ring. In sugars, this is referred to an aldose. Alternatively, if the hydroxyl attacks a ketone to produce a hemiketal, the resultant product is a 5-membered ring called a ketose, or furanose. Another convention to note is ‘D- ‘and ‘L- ‘.
The prefix ‘D- ‘is used to refer to the direction that an optical isomer (one with asymmetric groups surrounding a central atom) rotates plane polarised light. ‘D- ‘isomers have the OH of the chiral (C5) centre pointing to the right; ‘L- ‘isomers have the OH of the chiral (C5) centre pointing to the left.
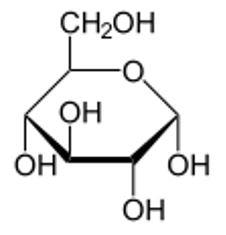
Figure 2 Haworth projection of α-D-Glucopyranose. Note the OH is on the opposite side of CH2OH
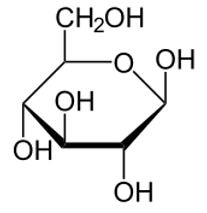
Figure 3 Haworth projection of β-D-Glucopyranose. Note the OH is on the same side as CH2OH.
Keto-Enol Intermediates in Sugar Interconversion and Epimerization
In sugars, the linear and cyclic hemiketal or hemiacetal of the sugar exist in equilibrium; in the linear form, sugars can undergo keto-enol tautomerism. This takes place during the interconversion between the aldose and ketose forms. Recall that tautomers are readily interconvertible isomers that differ in the position of the protons and electrons.
To convert between a 6-membered aldose, the sugar must tautomerise, to give an intermediate called and ene–diol, so-called because there is an alcohol group adjacent to the carbonyl (diol, two alcohols). This requires the presence of a base.
The base removes the proton adjacent to the anomeric, carbonyl carbon. This is referred to as the alpha hydrogen. In doing so, a double bond between the alpha carbon and carbonyl carbon is formed, as one of the C=O carbonyl bonds break, and the liberated electron pair on oxygen picks up a proton from an acid. This amounts to the movement of a proton from the alpha position to the carbonyl oxygen. Figure 4 is an example of D-glucose tautomerization.
The collapse to a ketose sugar requires the abstraction (removal) of the C2 (alpha) hydroxyl hydrogen by an acid, which subsequently results in the movement of the electron pair to the C2 carbon to form a carbonyl group.
One bond of the C=C bond opens to accommodate a hydrogen, provided by a base. The intramolecular reaction that occurs between the C5 carbon and C1 ketone results in the cyclic ketose.
The reaction is reversible; the proton is transferred from the C1 OH group along the C=C bond to the C2 alpha position. Figure 4 illustrates the mechanism of D-glucose tautomerization.
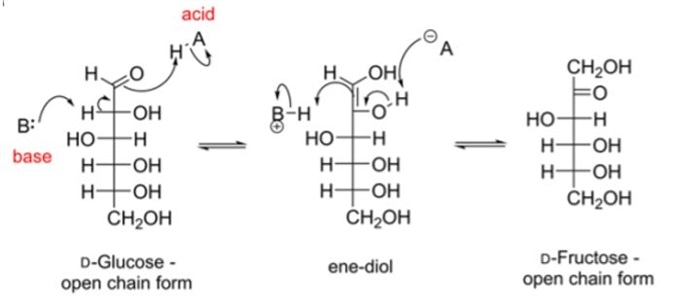
Figure 4 An enediol rearrangement. In the presence of a base, D-glucose may be converted to D-fructose. note how the position of the carbonyl has moved from C1 in D-glucose to C2 in D-fructose (movement of a bonding pair of electrons), and the alpha hydrogen has moved to C1 in the fructose/ ketose sugar.
The conjugate base of the enediol, called an enolate may also serve as an intermediate for another reaction called epimerisation. Epimers are optical isomers, differing in the arrangement of the same atoms about the anomeric carbon.
In this process, the enediol, in its deprotonated form called an enolate (the C1 hydroxyl lacks a proton), abstracts a proton from the neighbouring C2 hydroxyl.
The resulting deprotonated C2 oxygen donates a lone electron pair to the C2 carbon and thus form a carbonyl at this position. This is concurrent with breakage of one of the C=C bonds; the electron pair is used to accept a proton. This is illustrated in Figure 5.
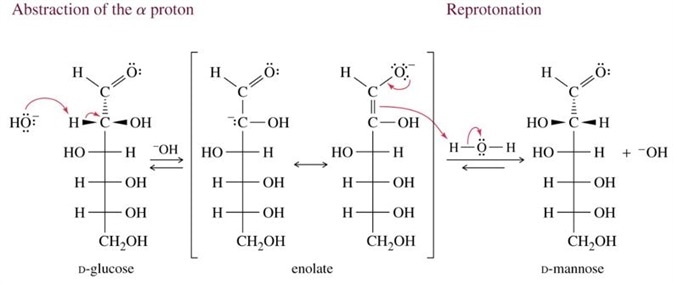
Figure 5 An Epimerisation reaction. This reaction intermediate is an enolate, the conjugate base of the enediol. In this example, D-glucose may be converted into D-mannose via the removal of hydrogen at C2 carbon followed by addition of hydrogen across the C=C of the enolate.
Keto-enol tautomerism is an important process in sugar biochemistry. The interconversion of an aldose to a ketose, such as D- glucose to D-fructose, occurs via their common enolate isomer. This is also true of epimerisation reactions, that allow interconversion of two aldoses, such as D-glucose and D-mannose or two ketoses, such as D-psicose and D-fructose. Epimers differ only in the arrangement of atoms or groups around the optically active, asymmetric C1.
Further Reading
Last Updated: Oct 30, 2018